Positive End-Expiratory Pressure Ventilation Induces Longitudinal Atrophy in Diaphragm Fibers
- PMID: 29578749
- PMCID: PMC6118031
- DOI: 10.1164/rccm.201709-1917OC
Positive End-Expiratory Pressure Ventilation Induces Longitudinal Atrophy in Diaphragm Fibers
Abstract
Rationale: Diaphragm weakness in critically ill patients prolongs ventilator dependency and duration of hospital stay and increases mortality and healthcare costs. The mechanisms underlying diaphragm weakness include cross-sectional fiber atrophy and contractile protein dysfunction, but whether additional mechanisms are at play is unknown.
Objectives: To test the hypothesis that mechanical ventilation with positive end-expiratory pressure (PEEP) induces longitudinal atrophy by displacing the diaphragm in the caudal direction and reducing the length of fibers.
Methods: We studied structure and function of diaphragm fibers of mechanically ventilated critically ill patients and mechanically ventilated rats with normal and increased titin compliance.
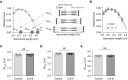
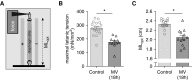
Measurements and main results: PEEP causes a caudal movement of the diaphragm, both in critically ill patients and in rats, and this caudal movement reduces fiber length. Diaphragm fibers of 18-hour mechanically ventilated rats (PEEP of 2.5 cm H2O) adapt to the reduced length by absorbing serially linked sarcomeres, the smallest contractile units in muscle (i.e., longitudinal atrophy). Increasing the compliance of titin molecules reduces longitudinal atrophy.
Conclusions: Mechanical ventilation with PEEP results in longitudinal atrophy of diaphragm fibers, a response that is modulated by the elasticity of the giant sarcomeric protein titin. We postulate that longitudinal atrophy, in concert with the aforementioned cross-sectional atrophy, hampers spontaneous breathing trials in critically ill patients: during these efforts, end-expiratory lung volume is reduced, and the shortened diaphragm fibers are stretched to excessive sarcomere lengths. At these lengths, muscle fibers generate less force, and diaphragm weakness ensues.
Keywords: PEEP; critically ill; diaphragm; mechanical ventilation.
Figures





Comment in
-
Diaphragm Remodeling during Application of Positive End-Expiratory Pressure. A Case of Normal Physiologic Adaptation Gone Awry?Am J Respir Crit Care Med. 2018 Aug 15;198(4):416-418. doi: 10.1164/rccm.201803-0518ED. Am J Respir Crit Care Med. 2018. PMID: 29589765 No abstract available.
References
-
- Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wendon J, et al. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med. 2001;29:1325–1331. - PubMed
-
- Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183:364–371. - PubMed
-
- Gottesman E, McCool FD. Ultrasound evaluation of the paralyzed diaphragm. Am J Respir Crit Care Med. 1997;155:1570–1574. - PubMed
-
- Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of diaphragm thickness during mechanical ventilation. impact of inspiratory effort. Am J Respir Crit Care Med. 2015;192:1080–1088. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

