Pilot Randomized Trial of an Automated Smoking Cessation Intervention via Mobile Phone Text Messages as an Adjunct to Varenicline in Primary Care
- PMID: 29578832
- PMCID: PMC11181465
- DOI: 10.1080/10810730.2018.1453890
Pilot Randomized Trial of an Automated Smoking Cessation Intervention via Mobile Phone Text Messages as an Adjunct to Varenicline in Primary Care
Abstract
Objective: Varenicline is a safe and effective aid to smoking cessation but most trials have involved frequent visits or intensive behavioral support unlike that typically provided in primary care. The current study examined if motivational text messages, sent via cellphone, would increase quit rates in smokers being treated with varenicline and 3 brief sessions in a family practice setting.
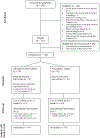
Methods: This study was a randomized controlled, parallel-group smoking cessation trial. Intervention group participants (n = 74) received daily motivational text messages, additional texted tips in response to keywords, and weekly study questions while control group participants (n = 76) received only weekly study questions. Both groups received individualized counseling. Self-reported non-smoking and exhaled breath CO <10ppm were used to validate smoking abstinence at 3 weeks and 12 weeks.
Results: Overall, 30.7% (46/150) of participants were abstinent at the 12 week follow-up and the abstinence rate did not differ between groups (INT 31.1% v. CON 30.3%, p = .91). The only predictor of abstinence at 12 weeks was use of varenicline during a previous quit attempt (p = .01). Intervention group participants were more likely to rate the text messaging program as good or excellent (p < .01), to recommend a similar program to family or friends (p < .01), and to complete positive smoking cessation activities (p = .04), when compared with the control group.
Conclusion: Although there were no differences in quit rates between the intervention and control group, intervention group participants rated the text messaging system more favorably, were more likely to recommend the program to others, and were more likely to complete positive smoking cessation activities.
Trial registration: ClinicalTrials.gov NCT02367391.
Conflict of interest statement
JF has done paid consulting work for pharmaceutical companies that manufacture or develop smoking cessation medicines (including Pfizer, GSK, J&J). The authors are primarily funded by grants from the National Institutes of Health (NIDA).
References
-
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, … Evins AE (2016). Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): A double-blind, randomised, placebo-controlled clinical trial. Lancet, 387(10037), 2507–2520. doi:10.1016/S0140-6736(16)30272-0 - DOI - PubMed
-
- Brandon TH, Meade CD, Herzog TA, Chirikos TN, Webb MS, & Cantor AB (2004). Efficacy and cost-effectiveness of a minimal intervention to prevent smoking relapse: Dismantling the effects of amount of content versus contact. Journal of Consulting and Clinical Psychology, 72(5), 797–808. doi:10.1037/0022-006X.72.5.797 - DOI - PubMed
-
- Eriksen M, Mackay J, Schulger N, Gomeshtapeh F, & Drope J (2015). The tobacco atlas (5th ed.). Atlanta, Georgia: American Cancer Society, Inc.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical