PrePex circumcision surveillance: Adverse events and analgesia for device removal
- PMID: 29579082
- PMCID: PMC5868790
- DOI: 10.1371/journal.pone.0194271
PrePex circumcision surveillance: Adverse events and analgesia for device removal
Abstract
Background: The PrePex medical male circumcision (MMC) device is relatively easy to place and remove with some training. PrePex has been evaluated in several countries to assess feasibility and acceptability. However, several studies have reported pain associated with removal.
Objective: To assess safety of PrePex and whether analgesia administered prior to removal reduces pain experienced by participants.
Methods: A multi-site non-randomized, prospective cohort study in which adult (18-45 years old) males requesting PrePex device male circumcision, were enrolled in six South African clinics from July 2014 to March 2015. Participants were routinely provided with analgesia shortly after the surveillance commenced following a protocol review. Analgesia regimen for device removal depended on medication availability at clinics.
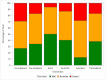
Results: Of 1023 enrolled participants who had PrePex placed, 98% (1004) had the device removed at a study clinic. Their median age was 25 (IQR: 21-30) years. HIV sero-positivity was 3.6% (37/1023). Nurses placed and removed half of all devices. Adverse events were experienced by 2.4% (25/1023) of participants; 15 required surgical intervention: device displacement (5/14), early removals (3/14), self-removals (5/14) and insufficient skin removed (2/14). Majority (792: 79%) of participants received analgesia. Most received either paracetamol-codeine (33%), lidocaine (29%) or EMLA and Oral Combination (28%). A lower proportion of participants who received any analgesia (except for lidocaine) prior to PrePex removal experienced severe pain compared to those who received no analgesia (16.6% vs. 29%: p = 0.0001).
Conclusion: Reported adverse events during this PrePex active surveillance were similar to previous reports and to those of surgical circumcision. Pain medication provided prior to removal is effective at decreasing severe pain during PrePex device removal.
Conflict of interest statement
Figures
References
-
- UNAIDS. HIV and AIDS Estimates 2015 South Africa 2015 [cited 2017 21 November]. Available from: http://www.unaids.org/en/regionscountries/countries/southafrica.
-
- UNAIDS. New HIV report finds big drop in new HIV infections in South Africa. 2014 [cited 2017 21 November ]. Available from: http://www.unaids.org/en/resources/presscentre/featurestories/2014/janua....
-
- World Health Organisation. PrePex device for adult male circumcision for HIV prevention 2013 [cited 2017 21 November]. Available from: http://www.who.int/hiv/topics/malecircumcision/prepex_device_update/en/
-
- World Health Organisation. Voluntary Medical Male Circumcision for circumcision in 14 priority countries in East and Southern Africa 2015 [cited 2017 21 November ]. Available from: http://www.who.int/hiv/pub/malecircumcision/brief2015/en/
-
- Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11):e298 doi: 10.1371/journal.pmed.0020298 ; PubMed Central PMCID: PMCPMC1262556. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical