Data-driven models of dominantly-inherited Alzheimer's disease progression
- PMID: 29579160
- PMCID: PMC5920320
- DOI: 10.1093/brain/awy050
Data-driven models of dominantly-inherited Alzheimer's disease progression
Abstract
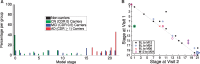
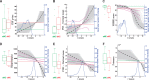
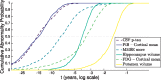
See Li and Donohue (doi:10.1093/brain/awy089) for a scientific commentary on this article.Dominantly-inherited Alzheimer's disease is widely hoped to hold the key to developing interventions for sporadic late onset Alzheimer's disease. We use emerging techniques in generative data-driven disease progression modelling to characterize dominantly-inherited Alzheimer's disease progression with unprecedented resolution, and without relying upon familial estimates of years until symptom onset. We retrospectively analysed biomarker data from the sixth data freeze of the Dominantly Inherited Alzheimer Network observational study, including measures of amyloid proteins and neurofibrillary tangles in the brain, regional brain volumes and cortical thicknesses, brain glucose hypometabolism, and cognitive performance from the Mini-Mental State Examination (all adjusted for age, years of education, sex, and head size, as appropriate). Data included 338 participants with known mutation status (211 mutation carriers in three subtypes: 163 PSEN1, 17 PSEN2, and 31 APP) and a baseline visit (age 19-66; up to four visits each, 1.1 ± 1.9 years in duration; spanning 30 years before, to 21 years after, parental age of symptom onset). We used an event-based model to estimate sequences of biomarker changes from baseline data across disease subtypes (mutation groups), and a differential equation model to estimate biomarker trajectories from longitudinal data (up to 66 mutation carriers, all subtypes combined). The two models concur that biomarker abnormality proceeds as follows: amyloid deposition in cortical then subcortical regions (∼24 ± 11 years before onset); phosphorylated tau (17 ± 8 years), tau and amyloid-β changes in cerebrospinal fluid; neurodegeneration first in the putamen and nucleus accumbens (up to 6 ± 2 years); then cognitive decline (7 ± 6 years), cerebral hypometabolism (4 ± 4 years), and further regional neurodegeneration. Our models predicted symptom onset more accurately than predictions that used familial estimates: root mean squared error of 1.35 years versus 5.54 years. The models reveal hidden detail on dominantly-inherited Alzheimer's disease progression, as well as providing data-driven systems for fine-grained patient staging and prediction of symptom onset with great potential utility in clinical trials.
Figures




Comment in
-
Disease progression models for dominantly-inherited Alzheimer's disease.Brain. 2018 May 1;141(5):1244-1246. doi: 10.1093/brain/awy089. Brain. 2018. PMID: 29701790 Free PMC article.
References
-
- Berg L. Clinical Dementia Rating (CDR). Psychopharmacol Bull 1988; 24: 637–9. - PubMed
-
- Cairns NJ, Perrin RJ, Franklin EE, Carter D, Vincent B, Xie M, et al.Neuropathologic assessment of participants in two multi-center longitudinal observational studies: the Alzheimer Disease Neuroimaging Initiative (ADNI) and the Dominantly Inherited Alzheimer Network (DIAN). Neuropathology 2015; 35: 390–400. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AG032438/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- MR/L023784/1/MRC_/Medical Research Council/United Kingdom
- MR/009076/2/MRC_/Medical Research Council/United Kingdom
- UF1 AG032438/AG/NIA NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- MR/L023784/2/MRC_/Medical Research Council/United Kingdom
- MR/M023664/1/MRC_/Medical Research Council/United Kingdom
- UL1 TR002345/TR/NCATS NIH HHS/United States
- U19 AG032438/AG/NIA NIH HHS/United States
- MR/M009106/1/MRC_/Medical Research Council/United Kingdom
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

