MiR-135a-5p represses proliferation of HNSCC by targeting HOXA10
- PMID: 29580143
- PMCID: PMC6301828
- DOI: 10.1080/15384047.2018.1450112
MiR-135a-5p represses proliferation of HNSCC by targeting HOXA10
Abstract
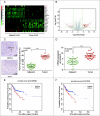
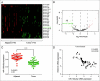
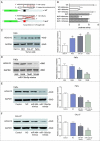
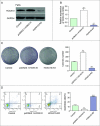
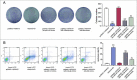
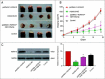
Objectives: This research aimed to explore the role of miR-135a-5p in head and neck squamous cell carcinoma (HNSCC) cells and its influence on cell viability. Moreover, we aimed to compare effects of miR-135a-5p and miR-494 in HNSCC, which was found to repress HOXA10 expression in oral cancer. Methods: The association between miR-135a-5p and HOXA10 was confirmed by green fluorescence protein reporter assay and qRT-PCR. The expression levels of HOXA10 in HNSCC cell lines (CAL-27, FaDu and NEC) were examined using western blot. The expression levels of HOXA10 in FaDu cells and CAL-27 cells were examined by western blot after transfection with miR-135a-5p mimics and miR-494 mimics. Colony formation assay and flow cytometry assay were respectively utilized to detect the proliferation and apoptosis of HNSCC cells after transfection with HOXA10 plasmids and HOXA10-KO plasmids. In vitro tumor xenograft experiments were performed to analyze the inhibitive effect of miR-135a-5p on HOXA10 in BALA/c mice. Results: HOXA10 was overexpressed in HNSCC cells, while miR-135a-5p was under-expressed. Therefore, low expression of HOXA10 lengthened disease-free survival time and overall survival time. MiR-135a-5p overexpression could inhibit HOXA10 expression by directly targeting HOXA10 3'UTR, and the inhibition was more effective than miR-494. HOXA10 suppression inhibited proliferation and enhanced apoptosis of HNSCC cells. In vivo experiments showed that miR-135a-5p could decelerate the growth of tumor cells in mice by downregulating HOXA10 expression. Conclusion: MiR-135a-5p could repress HNSCC cells proliferation and enhance apoptosis by directly targeting HOXA10, implying miR-135a-5p's significance on HNSCC treatment.
Keywords: HNSCC; miR-135a-5p; miR-494.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
