Evaluation of a program for routine implementation of shared decision-making in cancer care: study protocol of a stepped wedge cluster randomized trial
- PMID: 29580249
- PMCID: PMC5870914
- DOI: 10.1186/s13012-018-0740-y
Evaluation of a program for routine implementation of shared decision-making in cancer care: study protocol of a stepped wedge cluster randomized trial
Abstract
Background: Shared decision-making (SDM) has become increasingly important in health care. However, despite scientific evidence, effective implementation strategies, and a prominent position on the health policy agenda, SDM is not widely implemented in routine practice so far. Therefore, we developed a program for routine implementation of SDM in oncology by conducting an analysis of the current state and a needs assessment in a pilot study based on the Consolidated Framework for Implementation Research (CFIR). Based on these results, the main aim of our current study is to evaluate the process and outcome of this theoretically and empirically grounded multicomponent implementation program designed to foster SDM in routine cancer care.
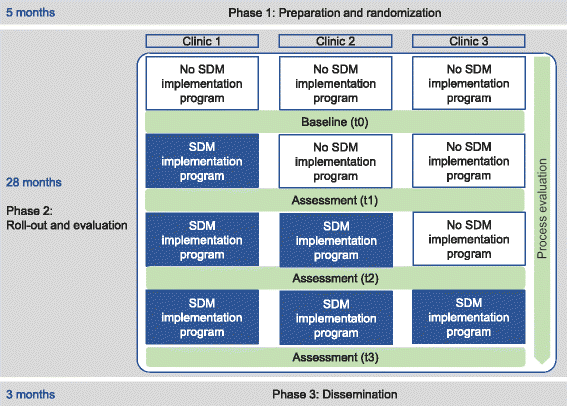
Methods: We use a stepped wedge design, a variant of the cluster randomized controlled trial. The intervention to be implemented is SDM. Three participating clinics of one comprehensive cancer center will be randomized and receive the multicomponent SDM implementation program in a time-delayed sequence. The program consists of the following strategies: (a) SDM training for health care professionals, (b) individual coaching for physicians, (c) patient activation strategy, (d) provision of patient information material and decision aids, (e) revision of the clinics' quality management documents, and (f) critical reflection of current organization of multidisciplinary team meetings. We will conduct a mixed methods outcome and process evaluation. The outcome evaluation will consist of four measurement points. The primary outcome is adoption of SDM, measured by the 9-item Shared Decision Making Questionnaire. A range of other implementation outcomes will be assessed (i.e., acceptability, readiness for implementing change, appropriateness, penetration). The implementation process will be evaluated using stakeholder interviews and field notes. This will allow adapting interventions if necessary.
Discussion: This study is the first large study on routine implementation of SDM conducted in German cancer care. We expect to foster implementation of SDM at the enrolled clinics. Insights gained from this study, using a theoretically and empirically grounded approach, can inform other SDM implementation studies and health policy developments, both nationally and internationally.
Trial registration: clinicaltrials.gov, NCT03393351 . Registered 8 January 2018.
Keywords: Cancer; Cluster randomized controlled trial; Health services research; Implementation science; Shared decision-making; Stepped wedge design.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Medical Association Hamburg (Germany, study ID PV5368). The study will be carried out in accordance with the latest version of the Helsinki Declaration of the World Medical Association. Principles of good clinical practice will be respected. Data protection requirements will be met. For patients that are participating in data collection (i.e., audio recording of consultations, filling in SDM-Q-9), written consent will be obtained after information on aims of the study, data collection, and the use of collected data. Study participation is voluntary. A waiver of consent for HCPs was obtained from the ethics committee, as proposed by current statements on ethical designs of cluster randomized trials [78] and implementation research [79]. All participating HCPs may decline to participate in the study.
Consent for publication
Not applicable.
Competing interests
IS’s, PH’s, AL’s, LK’s, and MH’s institution receives the grant from the German Research Foundation for the study described in this study protocol (cp. funding). IS conducted one physician training in shared decision-making for which she received travel compensation from Mundipharma GmBH in 2015. MH declares that he is a co-PI in SDM training projects, one funded by the German Cancer Aid and one by Mundipharma. CB is the president of the German Society for Hematology and Oncology and receives support for clinical trials from several pharmaceutical companies (> 70 trails in his department) and holds grants, e.g., from the German Cancer Aid, Hubertus Wald Foundation and others. The authors did not receive industry funding for this paper, nor were pharmaceutical companies involved in any steps of the study or publication process. PH, AL, AC, HH, VM, RS, IW and LK declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Coulter A, Magee H. The European patient of the future. Maidenhead: Open University Press; 2003.
-
- Mühlhauser I, Steckelberg A. Evidence-based patient information: preferences of patients [German] Dtsch Arztebl. 2009;51–52:2554–2556.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical