Comparison of an injectable toltrazuril-gleptoferron (Forceris®) and an oral toltrazuril (Baycox®) + injectable iron dextran for the control of experimentally induced piglet cystoisosporosis
- PMID: 29580269
- PMCID: PMC5870915
- DOI: 10.1186/s13071-018-2797-5
Comparison of an injectable toltrazuril-gleptoferron (Forceris®) and an oral toltrazuril (Baycox®) + injectable iron dextran for the control of experimentally induced piglet cystoisosporosis
Abstract
Background: Cystoisospora suis causes diarrhoeal disease and reduced weight gain in suckling piglets, and a toltrazuril-based oral suspension is available for treatment. Recently a combinatorial product with toltrazuril plus iron has been developed for parenteral application. In this study we compared the efficacy of the injectable product with the oral suspension against experimentally induced piglet cystoisosporosis.
Methods: In a randomised controlled study, three groups of piglets (n = 10-13) were treated either with a fixed dose of 45 mg toltrazuril + 200 mg gleptoferron i.m. per piglet (Forceris®) on the second day of life (study day 2; SD 2) or with 20 mg toltrazuril/kg body weight as an oral suspension (Baycox® 5%) on SD 4 or left untreated (Control group). The Baycox® and the Control group received 200 mg of iron dextran/piglet on SD 2. All piglets were infected with 1000 sporulated C. suis oocysts on SD 3. Faecal samples were taken daily from SD 7 to SD 20 to determine faecal consistency, oocyst shedding and other diarrhoeal pathogens. Body weight was recorded on SD 1 and then weekly until SD 29. Animals were observed daily for general health and after treatment for possible adverse events.
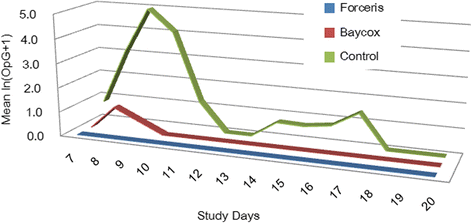
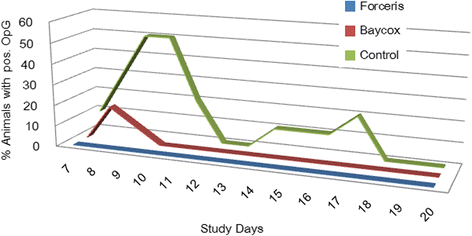
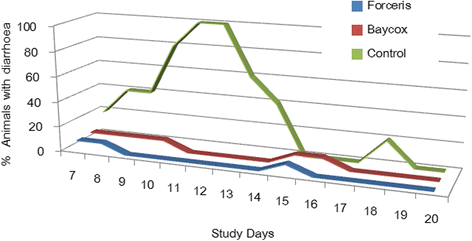
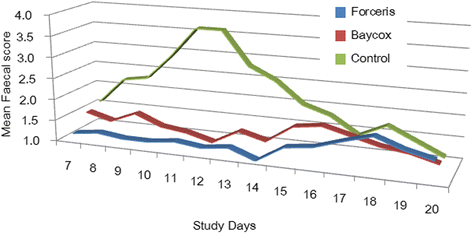
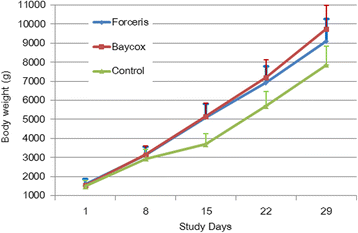
Results: In the Control group all animals shed oocysts for 3.1 days on average and all animals showed diarrhoea for an average of five days. Excretion peaked on SD 9 (max. 48,618 oocysts per gram of faeces). Treatment with Forceris® completely suppressed oocyst excretion. In the Baycox® group, low levels of excretion could be detected. Diarrhoea was reduced to single piglets in the treated groups. Body weight development was reduced in the Control group compared to the treated groups. Enteropathogenic bacteria (Escherichia coli, Clostridium perfringens) could be detected. All parameters related to oocyst excretion, faecal consistency and weight gain were significantly improved in the treated groups compared to the Control group without significant differences between the treated groups. Both products were safe to use.
Conclusions: Treatment with both the injectable (Forceris®) and the oral (Baycox®) formulation of toltrazuril in the prepatent period were safe and highly effective against experimental infection with C. suis in newborn piglets.
Keywords: Coccidiosis; Cystoisospora suis; Diarrhoea; Efficacy; Experimental infection; Isospora suis; Pig; Swine; Toltrazuril.
Conflict of interest statement
Ethics approval
The procedures involving piglets for collection of oocysts were approved by the institutional ethics committee and the national authority according to § 26ff of Animal Experiments Act, Tierversuchsgesetz 2012-TVG 2012 (license number: BMWF-68.205/0034-WF/V/3b/2016; Austrian Federal Ministry of Science, Health and Economy).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. DS and HK are employees of Ceva. No member of the staff of the Vetmeduni Vienna involved in the trial received allowances or other personal benefits from the Sponsor.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Efficacy of injectable toltrazuril-iron combination product and oral toltrazuril against early experimental infection of suckling piglets with Cystoisospora suis.Parasit Vectors. 2019 May 28;12(1):272. doi: 10.1186/s13071-019-3527-3. Parasit Vectors. 2019. PMID: 31138327 Free PMC article.
-
The effect of an injectable toltrazuril - gleptoferron (Forceris®) on Cystoisospora suis oocyst excretion and growth of neonatal piglets pre- and post-weaning.Vet Parasitol. 2024 Jun;328:110179. doi: 10.1016/j.vetpar.2024.110179. Epub 2024 Mar 31. Vet Parasitol. 2024. PMID: 38579607
-
Experimentally confirmed toltrazuril resistance in a field isolate of Cystoisospora suis.Parasit Vectors. 2017 Jun 29;10(1):317. doi: 10.1186/s13071-017-2257-7. Parasit Vectors. 2017. PMID: 28662668 Free PMC article.
-
Isospora suis infection in piglets. A review.Vet Q. 1983 Oct;5(4):178-85. doi: 10.1080/01652176.1983.9693894. Vet Q. 1983. PMID: 6359663 Review.
-
Prevalence of coccidia in domestic pigs in China between 1980 and 2019: a systematic review and meta-analysis.Parasit Vectors. 2021 May 10;14(1):248. doi: 10.1186/s13071-021-04611-x. Parasit Vectors. 2021. PMID: 33971953 Free PMC article.
Cited by
-
Pharmacokinetics, bioavailability, and excretion of ponazuril in piglets.Front Vet Sci. 2022 Dec 7;9:1054417. doi: 10.3389/fvets.2022.1054417. eCollection 2022. Front Vet Sci. 2022. PMID: 36570513 Free PMC article.
-
Effect of intramuscular treatment with different iron dextran dosages and non-inferiority study to gleptoferron.Acta Vet Scand. 2025 Jan 4;67(1):1. doi: 10.1186/s13028-024-00790-6. Acta Vet Scand. 2025. PMID: 39755680 Free PMC article.
-
Comparative efficacy of two parenteral iron-containing preparations, iron gleptoferron and iron dextran, for the prevention of anaemia in suckling piglets.Vet Rec Open. 2018 Dec 5;5(1):e000317. doi: 10.1136/vetreco-2018-000317. eCollection 2018. Vet Rec Open. 2018. PMID: 30613406 Free PMC article.
-
Iron status in piglets at three days of age and at weaning and possible seasonal effects on the blood haemoglobin levels in a Swedish outdoor pig-producing farm.Acta Vet Scand. 2024 Mar 19;66(1):13. doi: 10.1186/s13028-024-00735-z. Acta Vet Scand. 2024. PMID: 38504355 Free PMC article.
-
Shifts in the Fecal Microbial Community of Cystoisospora suis Infected Piglets in Response to Toltrazuril.Front Microbiol. 2020 May 19;11:983. doi: 10.3389/fmicb.2020.00983. eCollection 2020. Front Microbiol. 2020. PMID: 32508791 Free PMC article.
References
-
- Lindsay DS, Current WL, Taylor JR. Effects of experimentally induced Isospora suis infection on morbidity, mortality, and weight gains in nursing pigs. Am J Vet Res. 1985;46(7):1511–1512. - PubMed
-
- Lindsay DS, Current WL, Power TA. Enteric coccidial infections and coccidiosis in swine. Compend Contin Educ Vet. 1992;14:698–702.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

