Hermansky-Pudlak syndrome type 2 manifests with fibrosing lung disease early in childhood
- PMID: 29580292
- PMCID: PMC5870397
- DOI: 10.1186/s13023-018-0780-z
Hermansky-Pudlak syndrome type 2 manifests with fibrosing lung disease early in childhood
Abstract
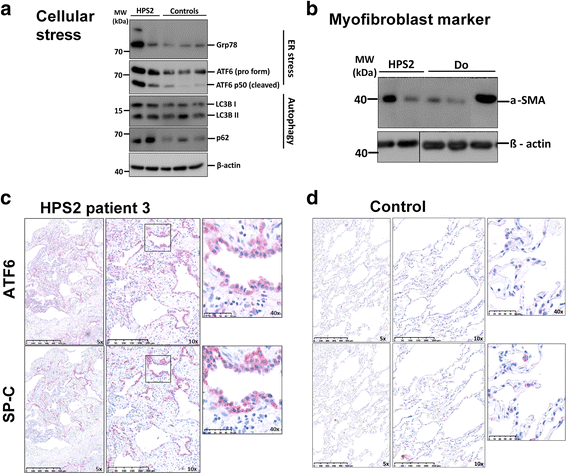
Background: Hermansky-Pudlak syndrome (HPS), a hereditary multisystem disorder with oculocutaneous albinism, may be caused by mutations in one of at least 10 separate genes. The HPS-2 subtype is distinguished by the presence of neutropenia and knowledge of its pulmonary phenotype in children is scarce.
Methods: Six children with genetically proven HPS-2 presented to the chILD-EU register between 2009 and 2017; the data were collected systematically and imaging studies were scored blinded.
Results: Pulmonary symptoms including dyspnea, coughing, need for oxygen, and clubbing started 3.3 years before the diagnosis was made at the mean age of 8.83 years (range 2-15). All children had recurrent pulmonary infections, 3 had a spontaneous pneumothorax, and 4 developed scoliosis. The frequency of pulmonary complaints increased over time. The leading radiographic pattern was ground-glass opacities with a rapid increase in reticular pattern and traction bronchiectasis between initial and follow-up Computer tomography (CT) in all subjects. Honeycombing and cysts were newly detectable in 3 patients. Half of the patients received a lung biopsy for diagnosis; histological patterns were cellular non-specific interstitial pneumonia, usual interstitial pneumonia-like, and desquamative interstitial pneumonia.
Conclusions: HPS-2 is characterized by a rapidly fibrosing lung disease during early childhood. Effective treatments are required.
Keywords: Childhood; Hermansky-Pudlak syndrome type 2; Pulmonary fibrosis; Pulmonary phenotype; Tachydyspnea.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the ethics committee of the Ludwig-Maximilians University of Munich (EK 111-13).
Consent for publication
Informed consent to report individual patient data was obtained by all patients old enough to consent, and their parents or guardians.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

