Analytical validation of an ultra low-cost mobile phone microplate reader for infectious disease testing
- PMID: 29580858
- PMCID: PMC6391724
- DOI: 10.1016/j.cca.2018.03.013
Analytical validation of an ultra low-cost mobile phone microplate reader for infectious disease testing
Abstract
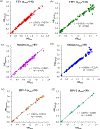
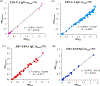
Most mobile health (mHealth) diagnostic devices for laboratory tests only analyze one sample at a time, which is not suitable for large volume serology testing, especially in low-resource settings with shortage of health professionals. In this study, we developed an ultra-low-cost clinically-accurate mobile phone microplate reader (mReader), and clinically validated this optical device for 12 infectious disease tests. The mReader optically reads 96 samples on a microplate at one time. 771 de-identified patient samples were tested for 12 serology assays for bacterial/viral infections. The mReader and the clinical instrument blindly read and analyzed all tests in parallel. The analytical accuracy and the diagnostic performance of the mReader were evaluated across the clinical reportable categories by comparison with clinical laboratorial testing results. The mReader exhibited 97.59-99.90% analytical accuracy and <5% coefficient of variation (CV). The positive percent agreement (PPA) in all 12 tests achieved 100%, negative percent agreement (NPA) was higher than 83% except for one test (42.86%), and overall percent agreement (OPA) ranged 89.33-100%. We envision the mReader can benefit underserved areas/populations and low-resource settings in rural clinics/hospitals at a low cost (~$50 USD) with clinical-level analytical quality. It has the potential to improve health access, speed up healthcare delivery, and reduce health disparities and education disparities by providing access to a low-cost spectrophotometer.
Keywords: Analytical validation; Infectious diseases; Microplate reader; Mobile health diagnostic device; Optical device; Serology tests.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures
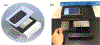
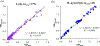
Similar articles
-
Measuring mobility, disease connectivity and individual risk: a review of using mobile phone data and mHealth for travel medicine.J Travel Med. 2019 May 10;26(3):taz019. doi: 10.1093/jtm/taz019. J Travel Med. 2019. PMID: 30869148 Free PMC article. Review.
-
An ultra-low-cost smartphone octochannel spectrometer for mobile health diagnostics.J Biophotonics. 2018 Aug;11(8):e201700382. doi: 10.1002/jbio.201700382. Epub 2018 Apr 29. J Biophotonics. 2018. PMID: 29603674 Free PMC article.
-
High-throughput and automated diagnosis of antimicrobial resistance using a cost-effective cellphone-based micro-plate reader.Sci Rep. 2016 Dec 15;6:39203. doi: 10.1038/srep39203. Sci Rep. 2016. PMID: 27976700 Free PMC article.
-
Thousand-fold fluorescent signal amplification for mHealth diagnostics.Biosens Bioelectron. 2014 Jan 15;51:1-7. doi: 10.1016/j.bios.2013.06.053. Epub 2013 Jul 17. Biosens Bioelectron. 2014. PMID: 23928092 Free PMC article.
-
mHealth and big-data integration: promises for healthcare system in India.BMJ Health Care Inform. 2019 Sep;26(1):e100071. doi: 10.1136/bmjhci-2019-100071. BMJ Health Care Inform. 2019. PMID: 31488497 Free PMC article. Review.
Cited by
-
Modern Tools for Rapid Diagnostics of Antimicrobial Resistance.Front Cell Infect Microbiol. 2020 Jul 15;10:308. doi: 10.3389/fcimb.2020.00308. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32760676 Free PMC article. Review.
-
Sample Preparation and Diagnostic Methods for a Variety of Settings: A Comprehensive Review.Molecules. 2021 Sep 18;26(18):5666. doi: 10.3390/molecules26185666. Molecules. 2021. PMID: 34577137 Free PMC article. Review.
-
The Current and Future Use of Telemedicine in Infectious Diseases Practice.Curr Infect Dis Rep. 2019 Oct 19;21(11):41. doi: 10.1007/s11908-019-0697-2. Curr Infect Dis Rep. 2019. PMID: 31630276 Review.
-
Measuring mobility, disease connectivity and individual risk: a review of using mobile phone data and mHealth for travel medicine.J Travel Med. 2019 May 10;26(3):taz019. doi: 10.1093/jtm/taz019. J Travel Med. 2019. PMID: 30869148 Free PMC article. Review.
-
Portable Colorimetric Device with Commercial Microplates for Quantitative Detection of Urine Biomarkers: Design, Development, and Clinical Evaluation.Biosensors (Basel). 2022 Sep 4;12(9):723. doi: 10.3390/bios12090723. Biosensors (Basel). 2022. PMID: 36140108 Free PMC article.
References
-
- Cohen S, et al., Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect. Control. Hosp. Epidemiol. 31 (2010) 431–455. - PubMed
-
- Low N, et al., Global control of sexually transmitted infections. The Lancet 368 (2006) 2001–2016. - PubMed
-
- Manzi M, et al., High acceptability of voluntary counselling and HIV‐testing but unacceptable loss to follow up in a prevention of mother‐to‐child HIV transmission programme in rural Malawi: scaling‐up requires a different way of acting. Trop. Med. Int. Health 10 (2005) 1242–1250. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

