Species-conserved SYNGAP1 phenotypes associated with neurodevelopmental disorders
- PMID: 29580901
- PMCID: PMC6128754
- DOI: 10.1016/j.mcn.2018.03.008
Species-conserved SYNGAP1 phenotypes associated with neurodevelopmental disorders
Abstract
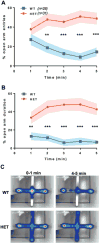
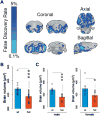
SYNGAP1 loss-of-function variants are causally associated with intellectual disability, severe epilepsy, autism spectrum disorder and schizophrenia. While there are hundreds of genetic risk factors for neurodevelopmental disorders (NDDs), this gene is somewhat unique because of the frequency and penetrance of loss-of-function variants found in patients combined with the range of brain disorders associated with SYNGAP1 pathogenicity. These clinical findings indicate that SYNGAP1 regulates fundamental neurodevelopmental processes that are necessary for brain development. Here, we describe four phenotypic domains that are controlled by Syngap1 expression across vertebrate species. Two domains, the maturation of cognitive functions and maintenance of excitatory-inhibitory balance, are defined exclusively through a review of the current literature. Two additional domains are defined by integrating the current literature with new data indicating that SYNGAP1/Syngap1 regulates innate survival behaviors and brain structure. These four phenotypic domains are commonly disrupted in NDDs, suggesting that a deeper understanding of developmental Syngap1 functions will be generalizable to other NDDs of known or unknown etiology. Therefore, we discuss the known molecular and cellular functions of Syngap1 and consider how these functions may contribute to the emergence of disease-relevant phenotypes. Finally, we identify major unexplored areas of Syngap1 neurobiology and discuss how a deeper understanding of this gene may uncover general principles of NDD pathobiology.
Keywords: Autism spectrum disorder; Circuits; Cognitive impairment; Epilepsy; Intellectual disability; Microcephaly; Neurodevelopment; SynGAP; Synapse; Syngap1.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

