Determination of the Dynamically Linked Indices of Fosfomycin for Pseudomonas aeruginosa in the Hollow Fiber Infection Model
- PMID: 29581114
- PMCID: PMC5971584
- DOI: 10.1128/AAC.02627-17
Determination of the Dynamically Linked Indices of Fosfomycin for Pseudomonas aeruginosa in the Hollow Fiber Infection Model
Abstract
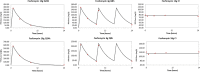
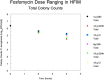
Fosfomycin is the only expoxide antimicrobial and is currently under development in the United States as an intravenously administered product. We were interested in identifying the exposure indices most closely linked to its ability to kill bacterial cells and to suppress amplification of less susceptible subpopulations. We employed the hollow fiber infection model for this investigation and studied wild-type strain Pseudomonas aeruginosa PAO1. Because of anticipated rapid resistance emergence, we shortened the study duration to 24 h but sampled the system more intensively. Doses of 12 and 18 g/day and schedules of daily administration, administration every 8 h, and administration by continuous infusion for each daily dose were studied. We measured fosfomycin concentrations (by liquid chromatography-tandem mass spectrometry), the total bacterial burden, and the burden of less susceptible isolates. We applied a mathematical model to all the data simultaneously. There was a rapid emergence of resistance with all doses and schedules. Prior to resistance emergence, an initial kill of 2 to 3 log10(CFU/ml) was observed. The model demonstrated that the area under the concentration-time curve/MIC ratio was linked to total bacterial kill, while the time that the concentration remained above the MIC (or, equivalently, the minimum concentration/MIC ratio) was linked to resistance suppression. These findings were also seen in other investigations with Enterobacteriaceae (in vitro systems) and P. aeruginosa (murine system). We conclude that for serious infections with high bacterial burdens, fosfomycin may be of value as a new therapeutic and may be optimized by administering the agent as a continuous or prolonged infusion or by use of a short dosing interval. For indications such as ventilator-associated bacterial pneumonia, it may be prudent to administer fosfomycin as part of a combination regimen.
Keywords: Pseudomonas aeruginosa; fosfomycin; hollow fiber infection model; pharmacodynamics.
Copyright © 2018 American Society for Microbiology.
Figures



References
-
- Docobo-Pérez F, Drusano GL, Johnson A, Goodwin J, Whalley S, Ramos-Martín V, Ballestero-Tellez M, Rodriguez-Martinez JM, Conejo C, van Guilder M, Rodríguez-Baño J, Pascual A, Hope WW. 2015. Pharmacodynamics of fosfomycin: insights into clinical use for antimicrobial resistance. Antimicrob Agents Chemother 59:5602–5610. doi:10.1128/AAC.00752-15. - DOI - PMC - PubMed
-
- VanScoy BD, McCauley J, Ellis-Grosse EJ, Okusanya OO, Bhavnani SM, Forrest A, Ambrose PG. 2015. Exploration of the pharmacokinetic-pharmacodynamic relationships for fosfomycin efficacy using an in vitro infection model. Antimicrob Agents Chemother 59:7170–7177. doi:10.1128/AAC.04955-14. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

