Inhibition of miR-34a-5p alleviates hypoxia-reoxygenation injury by enhancing autophagy in steatotic hepatocytes
- PMID: 29581146
- PMCID: PMC5898271
- DOI: 10.1242/bio.033290
Inhibition of miR-34a-5p alleviates hypoxia-reoxygenation injury by enhancing autophagy in steatotic hepatocytes
Abstract
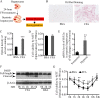
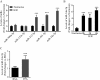
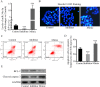
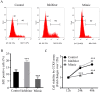
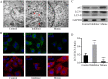
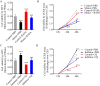
Hypoxia-reoxygenation (H/R) injury in steatotic hepatocytes has been implicated in liver dysfunction after liver transplantation. MicroRNAs (miRs) play important roles in regulating several cell biology mechanisms related to H/R injury. However, the role of miRs in regulating H/R injury in steatotic hepatocytes is still unclear. We established an in vitro model for studying H/R injury in steatotic hepatocytes and identified miR-34a-5p as a miR that was substantially upregulated in steatotic hepatocytes under H/R challenge. MiR-34a-5p expression was modified by transfecting miR-34a-5p mimic and inhibitor into H/R-challenged steatotic hepatocytes. We found that inhibition of miR-34a-5p alleviated H/R-induced apoptosis and promoted post-H/R proliferation in steatotic hepatocytes. Whereas, overexpression of miR-34a-5p augmented H/R-induced apoptosis and prohibited post-H/R proliferation. By examining autophagy, our data demonstrated that miR-34a-5p suppressed autophagy in H/R-challenged steatotic hepatocytes, induction of autophagy partially rescued the exaggeration of H/R injury induced by miR-34a-5p mimic, while inhibition of autophagy impaired the protection of the miR-34a-5p inhibitor against H/R injury. In conclusion, miR-34a-5p is crucial in exaggerating H/R injury, likely by suppressing autophagy in steatotic hepatocytes. Inhibition of miR-34a may be a promising strategy to protect steatotic hepatocytes against H/R-injury.
Keywords: Autophagy; Hypoxia/Reoxygenation injury; MiR-34a-5p; Steatosis.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

