Smoking abstinence 1 year after acute coronary syndrome: follow-up from a randomized controlled trial of varenicline in patients admitted to hospital
- PMID: 29581161
- PMCID: PMC5871438
- DOI: 10.1503/cmaj.170377
Smoking abstinence 1 year after acute coronary syndrome: follow-up from a randomized controlled trial of varenicline in patients admitted to hospital
Abstract
Background: Patients who continue to smoke after acute coronary syndrome are at increased risk of reinfarction and death. We previously found use of varenicline to increase abstinence 24 weeks after acute coronary syndrome; here we report results through 52 weeks.
Methods: The EVITA trial was a multicentre, double-blind, randomized, placebo-controlled trial of varenicline for smoking cessation in patients admitted to hospital with acute coronary syndrome. Participants were randomly assigned (1:1) to receive varenicline or placebo for 12 weeks, in conjunction with low-intensity counselling. Smoking abstinence was assessed via 7-day recall, with biochemical validation using exhaled carbon monoxide. Participants lost to follow-up or withdrawn were assumed to have returned to smoking.
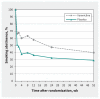
Results: Among the 302 participants, abstinence declined over the course of the trial, with 34.4% abstinent 52 weeks after acute coronary syndrome. Compared with placebo, point estimates suggest use of varenicline increased point-prevalence abstinence (39.9% v. 29.1%, difference 10.7%, 95% confidence interval [CI] 0.01% to 21.44%; number needed to treat 10), continuous abstinence (31.1% v. 21.2%, difference 9.9%, 95% CI -0.01% to 19.8%) and reduction in daily cigarette smoking by 50% or greater (57.8% v. 49.7%, difference 8.1%, 95% CI -3.1% to 19.4%). Varenicline and placebo groups had similar occurrence of serious adverse events (24.5% v. 21.9%, risk difference 2.7%, 95% CI -7.3% to 12.6%) and major adverse cardiovascular events (8.6% v. 9.3%, risk difference -0.7%, 95% CI -7.8% to 6.5%).
Interpretation: Varenicline was efficacious for smoking cessation in this high-risk patient population. However, 60% of patients who received treatment with varenicline still returned to smoking. Trial registration: ClinicalTrials.gov, no. NCT00794573.
© 2018 Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: Shamir Mehta reports funding from AstraZeneca, Boston Scientific, Bayer and Abbott. Beth Abramson has received grants or research support from AstraZeneca and Sanofi; honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol–Myers Squibb, Novartis, Fournier, Merck, Pfizer, Servier and Sanofi; and consulting fees from Amgen, Bayer, Boehringer Ingelheim, Sanofi and Servier. She authored Heart Health for Canadians. Mark Eisenberg, Payam Dehghani, François Grondin and Mina Madan received honoraria from Pfizer for providing continuing medical education on smoking cessation. No other competing interests were declared
Figures

Comment in
-
Tackling smoking cessation systematically among inpatients with heart disease.CMAJ. 2018 Mar 26;190(12):E345-E346. doi: 10.1503/cmaj.180125. CMAJ. 2018. PMID: 29581160 Free PMC article. No abstract available.
References
-
- Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 2003;290:86–97. - PubMed
-
- Colivicchi F, Mocini D, Tubaro M, et al. Effect of smoking relapse on outcome after acute coronary syndromes. Am J Cardiol 2011;108:804–8. - PubMed
-
- How tobacco smoking causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. - PubMed
-
- The health consequences of smoking: a report of the Surgeon General. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
-
- Dornelas EA, Sampson RA, Gray JF, et al. A randomized controlled trial of smoking cessation counseling after myocardial infarction. Prev Med 2000; 30:261–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
