Role of Sca2 and RickA in the Dissemination of Rickettsia parkeri in Amblyomma maculatum
- PMID: 29581194
- PMCID: PMC5964526
- DOI: 10.1128/IAI.00123-18
Role of Sca2 and RickA in the Dissemination of Rickettsia parkeri in Amblyomma maculatum
Abstract
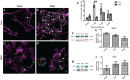
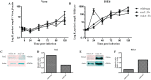
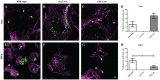
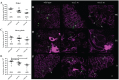
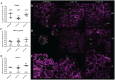
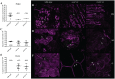
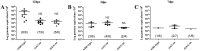
The Gram-negative obligate intracellular bacterium Rickettsia parkeri is an emerging tick-borne human pathogen. Recently, R. parkeri Sca2 and RickA have been implicated in adherence and actin-based motility in vertebrate host cell infection models; however, the rickettsia-derived factors essential to tick infection are unknown. Using R. parkeri mutants lacking functional Sca2 or RickA to compare actin polymerization, replication, and cell-to-cell spread in vitro, similar phenotypes in tick and mammalian cells were observed. Specifically, actin polymerization in cultured tick cells is controlled by the two separate proteins in a time-dependent manner. To assess the role of Sca2 and RickA in dissemination in the tick host, Rickettsia-free Amblyomma maculatum, the natural vector of R. parkeri, was exposed to wild-type, R. parkeri rickA::tn, or R. parkeri sca2::tn bacteria, and individual tick tissues, including salivary glands, midguts, ovaries, and hemolymph, were analyzed at 12 h and after continued bloodmeal acquisition for 3 or 7 days postexposure. Initially, ticks exposed to wild-type R. parkeri had the highest rickettsial load across all organs; however, rickettsial loads decreased and wild-type rickettsiae were cleared from the ovaries at 7 days postexposure. In contrast, ticks exposed to R. parkeririckA::tn or R. parkerisca2::tn had comparatively lower rickettsial loads, but bacteria persisted in all organs for 7 days. These data suggest that while RickA and Sca2 function in actin polymerization in tick cells, the absence of these proteins did not change dissemination patterns within the tick vector.
Keywords: Amblyomma maculatum; RickA; Rickettsia parkeri; Sca2; actin-based motility.
Copyright © 2018 Harris et al.
Figures


References
-
- Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, McLellan SL, Goddard J, Holman RC, Openshaw JJ, Sumner JW, Zaki SR, Eremeeva ME. 2008. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis 47:1188–1196. doi:10.1086/592254. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

