Kinetics and structural features of dimeric glutamine-dependent bacterial NAD+ synthetases suggest evolutionary adaptation to available metabolites
- PMID: 29581233
- PMCID: PMC5950007
- DOI: 10.1074/jbc.RA118.002241
Kinetics and structural features of dimeric glutamine-dependent bacterial NAD+ synthetases suggest evolutionary adaptation to available metabolites
Erratum in
-
Correction: Kinetics and structural features of dimeric glutamine-dependent bacterial NAD+ synthetases suggest evolutionary adaptation to available metabolites.J Biol Chem. 2019 Oct 11;294(41):15117. doi: 10.1074/jbc.AAC119.011035. Epub 2019 Oct 11. J Biol Chem. 2019. PMID: 31604837 Free PMC article. No abstract available.
Abstract
NADH (NAD+) and its reduced form NADH serve as cofactors for a variety of oxidoreductases that participate in many metabolic pathways. NAD+ also is used as substrate by ADP-ribosyl transferases and by sirtuins. NAD+ biosynthesis is one of the most fundamental biochemical pathways in nature, and the ubiquitous NAD+ synthetase (NadE) catalyzes the final step in this biosynthetic route. Two different classes of NadE have been described to date: dimeric single-domain ammonium-dependent NadENH3 and octameric glutamine-dependent NadEGln, and the presence of multiple NadE isoforms is relatively common in prokaryotes. Here, we identified a novel dimeric group of NadEGln in bacteria. Substrate preferences and structural analyses suggested that dimeric NadEGln enzymes may constitute evolutionary intermediates between dimeric NadENH3 and octameric NadEGln The characterization of additional NadE isoforms in the diazotrophic bacterium Azospirillum brasilense along with the determination of intracellular glutamine levels in response to an ammonium shock led us to propose a model in which these different NadE isoforms became active accordingly to the availability of nitrogen. These data may explain the selective pressures that support the coexistence of multiple isoforms of NadE in some prokaryotes.
Keywords: NAD biosynthesis; NadE; ammonia; ammonia assimilation; glutamine; glutamine synthase; nitrogen metabolism; nitrogenase.
© 2018 Santos et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




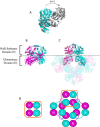
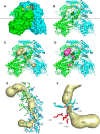
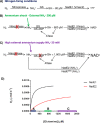
References
-
- Li J., Bonkowski M. S., Moniot S., Zhang D., Hubbard B. P., Ling A. J., Rajman L. A., Qin B., Lou Z., Gorbunova V., Aravind L., Steegborn C., and Sinclair D. A. (2017) A conserved NAD+ binding pocket that regulates protein–protein interactions during aging. Science 355, 1312–1317 10.1126/science.aad8242 - DOI - PMC - PubMed
-
- Moure V. R., Costa F. F., Cruz L. M., Pedrosa F. O., Souza E. M., Li X. D., Winkler F., and Huergo L. F. (2015) Regulation of nitrogenase by reversible mono-ADP-ribosylation. Curr. Top. Microbiol. Immunol. 384, 89–106 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

