Reduction of lipid accumulation rescues Bietti's crystalline dystrophy phenotypes
- PMID: 29581279
- PMCID: PMC5899444
- DOI: 10.1073/pnas.1717338115
Reduction of lipid accumulation rescues Bietti's crystalline dystrophy phenotypes
Abstract
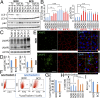
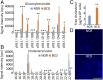
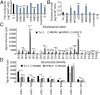
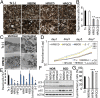
Bietti's crystalline dystrophy (BCD) is an intractable and progressive chorioretinal degenerative disease caused by mutations in the CYP4V2 gene, resulting in blindness in most patients. Although we and others have shown that retinal pigment epithelium (RPE) cells are primarily impaired in patients with BCD, the underlying mechanisms of RPE cell damage are still unclear because we lack access to appropriate disease models and to lesion-affected cells from patients with BCD. Here, we generated human RPE cells from induced pluripotent stem cells (iPSCs) derived from patients with BCD carrying a CYP4V2 mutation and successfully established an in vitro model of BCD, i.e., BCD patient-specific iPSC-RPE cells. In this model, RPE cells showed degenerative changes of vacuolated cytoplasm similar to those in postmortem specimens from patients with BCD. BCD iPSC-RPE cells exhibited lysosomal dysfunction and impairment of autophagy flux, followed by cell death. Lipidomic analyses revealed the accumulation of glucosylceramide and free cholesterol in BCD-affected cells. Notably, we found that reducing free cholesterol by cyclodextrins or δ-tocopherol in RPE cells rescued BCD phenotypes, whereas glucosylceramide reduction did not affect the BCD phenotype. Our data provide evidence that reducing intracellular free cholesterol may have therapeutic efficacy in patients with BCD.
Keywords: Bietti’s crystalline dystrophy; CYP4V2 gene; cholesterol; induced pluripotent stem cells; retinal pigment epithelium.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: Kyoto University has applied for patents related to this study (JP2017/90296) with M.H. and H.O.I. listed as inventors.
Figures

Similar articles
-
PSCs Reveal PUFA-Provoked Mitochondrial Stress as a Central Node Potentiating RPE Degeneration in Bietti's Crystalline Dystrophy.Mol Ther. 2020 Dec 2;28(12):2642-2661. doi: 10.1016/j.ymthe.2020.07.024. Epub 2020 Jul 25. Mol Ther. 2020. PMID: 32755565 Free PMC article.
-
Uncovering the role of ferroptosis in Bietti crystalline dystrophy and potential therapeutic strategies.Cell Commun Signal. 2024 Jul 11;22(1):359. doi: 10.1186/s12964-024-01710-x. Cell Commun Signal. 2024. PMID: 38992691 Free PMC article.
-
Targeted lipidomics uncovers oxylipin perturbations and potential circulation biomarkers in Bietti's crystalline dystrophy.Graefes Arch Clin Exp Ophthalmol. 2024 Dec;262(12):3773-3786. doi: 10.1007/s00417-024-06554-2. Epub 2024 Jul 4. Graefes Arch Clin Exp Ophthalmol. 2024. PMID: 38963460
-
Genetics of Bietti Crystalline Dystrophy.Asia Pac J Ophthalmol (Phila). 2016 Jul-Aug;5(4):245-52. doi: 10.1097/APO.0000000000000209. Asia Pac J Ophthalmol (Phila). 2016. PMID: 27228076 Review.
-
CYP4V2 fatty acid omega hydroxylase, a druggable target for the treatment of metabolic associated fatty liver disease (MAFLD).Biochem Pharmacol. 2022 Jan;195:114841. doi: 10.1016/j.bcp.2021.114841. Epub 2021 Nov 16. Biochem Pharmacol. 2022. PMID: 34798124 Review.
Cited by
-
Foveolar thickness as potential standardized structural outcome measurement in studies of Bietti crystalline dystrophy.Sci Rep. 2022 Aug 29;12(1):14706. doi: 10.1038/s41598-022-16563-y. Sci Rep. 2022. PMID: 36038562 Free PMC article.
-
A patient advocating for transparent science in rare disease research.Orphanet J Rare Dis. 2023 Jan 19;18(1):14. doi: 10.1186/s13023-022-02557-6. Orphanet J Rare Dis. 2023. PMID: 36658594 Free PMC article.
-
Relationship between outer retinal tubulation, retinal volume, and visual field in Bietti crystalline dystrophy.Graefes Arch Clin Exp Ophthalmol. 2025 Apr;263(4):993-1003. doi: 10.1007/s00417-025-06742-8. Epub 2025 Jan 31. Graefes Arch Clin Exp Ophthalmol. 2025. PMID: 39888431 Free PMC article.
-
DISCREPANCY BETWEEN FUNDUS AUTOFLUORESCENCE ABNORMALITY AND VISUAL FIELD LOSS IN BIETTI CRYSTALLINE DYSTROPHY.Retina. 2024 Aug 1;44(8):1394-1402. doi: 10.1097/IAE.0000000000004114. Retina. 2024. PMID: 39047130 Free PMC article.
-
Current perspectives in Bietti crystalline dystrophy.Clin Ophthalmol. 2019 Jul 30;13:1379-1399. doi: 10.2147/OPTH.S185744. eCollection 2019. Clin Ophthalmol. 2019. PMID: 31440027 Free PMC article.
References
-
- Bietti GB. Uber familiares Vorkommen von “retinitis punctata albescens” (verbunden mit “dystrophia marginalis cristallinza cornea”), Glitzern des Glaskorpers und anderen degenerativen Augenverunderungen. Klin Monatsbl Augenheilkd. 1937;99:737.
-
- Mataftsi A, Zografos L, Millá E, Secrétan M, Munier FL. Bietti’s crystalline corneoretinal dystrophy: a cross-sectional study. Retina. 2004;24:416–426. - PubMed
-
- Wilson DJ, Weleber RG, Klein ML, Welch RB, Green WR. Bietti’s crystalline dystrophy. A clinicopathologic correlative study. Arch Ophthalmol. 1989;107:213–221. - PubMed
-
- Halford S, et al. Detailed phenotypic and genotypic characterization of bietti crystalline dystrophy. Ophthalmology. 2014;121:1174–1184. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

