Sortase ligation enables homogeneous GPCR phosphorylation to reveal diversity in β-arrestin coupling
- PMID: 29581292
- PMCID: PMC5899476
- DOI: 10.1073/pnas.1722336115
Sortase ligation enables homogeneous GPCR phosphorylation to reveal diversity in β-arrestin coupling
Abstract
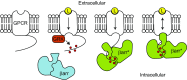
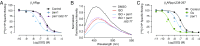
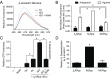
The ability of G protein-coupled receptors (GPCRs) to initiate complex cascades of cellular signaling is governed by the sequential coupling of three main transducer proteins, G protein, GPCR kinase (GRK), and β-arrestin. Mounting evidence indicates these transducers all have distinct conformational preferences and binding modes. However, interrogating each transducer's mechanism of interaction with GPCRs has been complicated by the interplay of transducer-mediated signaling events. For example, GRK-mediated receptor phosphorylation recruits and induces conformational changes in β-arrestin, which facilitates coupling to the GPCR transmembrane core. Here we compare the allosteric interactions of G proteins and β-arrestins with GPCRs' transmembrane cores by using the enzyme sortase to ligate a synthetic phosphorylated peptide onto the carboxyl terminus of three different receptors. Phosphopeptide ligation onto the β2-adrenergic receptor (β2AR) allows stabilization of a high-affinity receptor active state by β-arrestin1, permitting us to define elements in the β2AR and β-arrestin1 that contribute to the receptor transmembrane core interaction. Interestingly, ligation of the identical phosphopeptide onto the β2AR, the muscarinic acetylcholine receptor 2 and the μ-opioid receptor reveals that the ability of β-arrestin1 to enhance agonist binding relative to G protein differs substantially among receptors. Furthermore, strong allosteric coupling of β-arrestin1 correlates with its ability to attenuate, or "desensitize," G protein activation in vitro. Sortase ligation thus provides a versatile method to introduce complex, defined phosphorylation patterns into GPCRs, and analogous strategies could be applied to other classes of posttranslationally modified proteins. These homogeneously phosphorylated GPCRs provide an innovative means to systematically study receptor-transducer interactions.
Keywords: G protein-coupled receptor; allostery; phosphorylation; sortase; β-arrestin.
Conflict of interest statement
Conflict of interest statement: D.P.S., L.M.W., and R.J.L. are co-inventors on a patent application that covers methods described in this manuscript.
Figures



References
-
- Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. - PubMed
-
- Lefkowitz RJ. A brief history of G-protein coupled receptors (Nobel Lecture) Angew Chem Int Ed Engl. 2013;52:6366–6378. - PubMed
-
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

