Amide-forming chemical ligation via O-acyl hydroxamic acids
- PMID: 29581295
- PMCID: PMC5899448
- DOI: 10.1073/pnas.1718356115
Amide-forming chemical ligation via O-acyl hydroxamic acids
Abstract
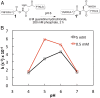
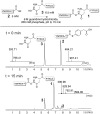
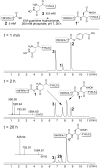
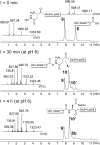
The facile rearrangement of "S-acyl isopeptides" to native peptide bonds via S,N-acyl shift is central to the success of native chemical ligation, the widely used approach for protein total synthesis. Proximity-driven amide bond formation via acyl transfer reactions in other contexts has proven generally less effective. Here, we show that under neutral aqueous conditions, "O-acyl isopeptides" derived from hydroxy-asparagine [aspartic acid-β-hydroxamic acid; Asp(β-HA)] rearrange to form native peptide bonds via an O,N-acyl shift. This process constitutes a rare example of an O,N-acyl shift that proceeds rapidly across a medium-size ring (t1/2 ∼ 15 min), and takes place in water with minimal interference from hydrolysis. In contrast to serine/threonine or tyrosine, which form O-acyl isopeptides only by the use of highly activated acyl donors and appropriate protecting groups in organic solvent, Asp(β-HA) is sufficiently reactive to form O-acyl isopeptides by treatment with an unprotected peptide-αthioester, at low mM concentration, in water. These findings were applied to an acyl transfer-based chemical ligation strategy, in which an unprotected N-terminal Asp(β-HA)-peptide and peptide-αthioester react under aqueous conditions to give a ligation product ultimately linked by a native peptide bond.
Keywords: O-acyl hydroxamic acid; acyl shift; acyl transfer; chemical ligation; hydroxamic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Schnölzer M, Kent SBH. Constructing proteins by dovetailing unprotected synthetic peptides: Backbone-engineered HIV protease. Science. 1992;256:221–225. - PubMed
-
- Kent SBH. Total chemical synthesis of proteins. Chem Soc Rev. 2009;38:338–351. - PubMed
-
- Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. - PubMed
-
- Rose K. Facile synthesis of homogeneous artificial proteins. J Am Chem Soc. 1994;116:30–33.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

