S-nitrosylation drives cell senescence and aging in mammals by controlling mitochondrial dynamics and mitophagy
- PMID: 29581312
- PMCID: PMC5899480
- DOI: 10.1073/pnas.1722452115
S-nitrosylation drives cell senescence and aging in mammals by controlling mitochondrial dynamics and mitophagy
Abstract
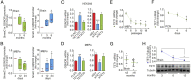
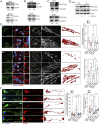
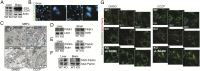
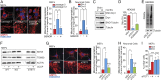
S-nitrosylation, a prototypic redox-based posttranslational modification, is frequently dysregulated in disease. S-nitrosoglutathione reductase (GSNOR) regulates protein S-nitrosylation by functioning as a protein denitrosylase. Deficiency of GSNOR results in tumorigenesis and disrupts cellular homeostasis broadly, including metabolic, cardiovascular, and immune function. Here, we demonstrate that GSNOR expression decreases in primary cells undergoing senescence, as well as in mice and humans during their life span. In stark contrast, exceptionally long-lived individuals maintain GSNOR levels. We also show that GSNOR deficiency promotes mitochondrial nitrosative stress, including excessive S-nitrosylation of Drp1 and Parkin, thereby impairing mitochondrial dynamics and mitophagy. Our findings implicate GSNOR in mammalian longevity, suggest a molecular link between protein S-nitrosylation and mitochondria quality control in aging, and provide a redox-based perspective on aging with direct therapeutic implications.
Keywords: GSNOR; S-nitrosylation; aging; mitochondria; mitophagy.
Conflict of interest statement
Conflict of interest statement: J.S.S. has received a commercial research grant from Novartis and has ownership interest (including patents) in Adamas and Lifehealth.
Figures


References
-
- Stamler JS, Lamas S, Fang FC. Nitrosylation. The prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. - PubMed
-
- Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

