Modeling the effects of lipid peroxidation during ferroptosis on membrane properties
- PMID: 29581451
- PMCID: PMC5979948
- DOI: 10.1038/s41598-018-23408-0
Modeling the effects of lipid peroxidation during ferroptosis on membrane properties
Abstract
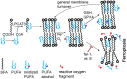
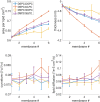
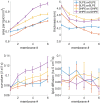
Ferroptosis is a form of regulated cell death characterized by the accumulation of lipid hydroperoxides. There has been significant research on the pathways leading to the accumulation of oxidized lipids, but the downstream effects and how lipid peroxides cause cell death during ferroptosis remain a major puzzle. We evaluated key features of ferroptosis in newly developed molecular dynamics models of lipid membranes to investigate the biophysical consequences of lipid peroxidation, and generated hypotheses about how lipid peroxides contribute to cell death during ferroptosis.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

