Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction
- PMID: 29581545
- PMCID: PMC5964317
- DOI: 10.1038/s41467-018-03566-5
Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction
Abstract
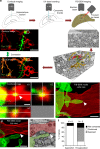
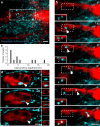
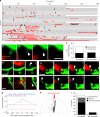
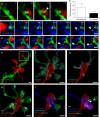
Microglia are highly motile glial cells that are proposed to mediate synaptic pruning during neuronal circuit formation. Disruption of signaling between microglia and neurons leads to an excess of immature synaptic connections, thought to be the result of impaired phagocytosis of synapses by microglia. However, until now the direct phagocytosis of synapses by microglia has not been reported and fundamental questions remain about the precise synaptic structures and phagocytic mechanisms involved. Here we used light sheet fluorescence microscopy to follow microglia-synapse interactions in developing organotypic hippocampal cultures, complemented by a 3D ultrastructural characterization using correlative light and electron microscopy (CLEM). Our findings define a set of dynamic microglia-synapse interactions, including the selective partial phagocytosis, or trogocytosis (trogo-: nibble), of presynaptic structures and the induction of postsynaptic spine head filopodia by microglia. These findings allow us to propose a mechanism for the facilitatory role of microglia in synaptic circuit remodeling and maturation.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
Synaptic nibbling.Nat Rev Neurosci. 2018 Jun;19(6):322. doi: 10.1038/s41583-018-0008-1. Nat Rev Neurosci. 2018. PMID: 29717204 No abstract available.
-
Microglia: Picky Brain Eaters.Dev Cell. 2019 Jan 7;48(1):3-4. doi: 10.1016/j.devcel.2018.12.013. Dev Cell. 2019. PMID: 30620901
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

