High expression of SLCO2B1 is associated with prostate cancer recurrence after radical prostatectomy
- PMID: 29581838
- PMCID: PMC5865664
- DOI: 10.18632/oncotarget.24453
High expression of SLCO2B1 is associated with prostate cancer recurrence after radical prostatectomy
Abstract
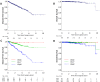
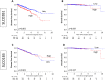
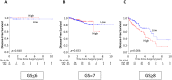
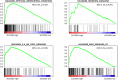
Solute carrier organic anion (SLCO) gene families encode organic anion transport proteins, which are transporters that up-take a number of substrates including androgens. Among them, high expression of SLCO2B1 is known to associate with the resistance to androgen deprivation therapy in prostate cancer (PCa). We hypothesized that high expression of SLCO genes enhances PCa progression by promoting the influx of androgen. Here, we demonstrated the impact of the expression levels of SLCO2B1 on prognosis in localized PCa after radical prostatectomy (RP) utilizing 494 PCa cases in The Cancer Genome Atlas (TCGA). SLCO2B1 high expression group showed significantly worse Disease-free survival (DFS) after RP (p = 0.001). The expression level of SLCO2B1 was significantly higher in advanced characteristics including Gleason Score (GS ≤ 6 vs GS = 7; p = 0.047, GS = 7 vs GS ≥ 8; p = 0.002), pathological primary tumor (pT2 vs pT3/4; p < 0.001), and surgical margin status (positive vs negative; p = 0.013), respectively. There was a significant difference in DFS between these two groups only in GS ≥ 8 patients (p = 0.006). Multivariate analysis demonstrated that only SLCO2B1 expression level was an independent predictor for DFS after RP in GS ≥ 8. SLCO2B1 high expressed tumors in GS ≥ 8 not only enriched epithelial mesenchymal transition (EMT) related gene set, (p = 0.027), as well as Hedgehog (p < 0.001), IL-6/JAK/STAT3 (p < 0.001), and K-ras signaling gene sets (p < 0.001), which are known to promote EMT, but also showed higher expression of EMT related genes, including N-cadherin (p = 0.024), SNAIL (p = 0.001), SLUG (p = 0.001), ZEB-1 (p < 0.001) and Vimentin (p < 0.001). In conclusion, PCa with high expression of SLCO2B1 demonstrated worse DFS, which might be due to accelerated EMT.
Keywords: EMT; OATP; SLCO2B1; prostate cancer; recurrence.
Conflict of interest statement
CONFLICTS OF INTEREST There are no potential conflicts of interest to disclose.
Figures


References
-
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel T, Zattoni F, European Association of U EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. - DOI - PubMed
-
- Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–65. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

