Protein Kinase G Induces an Immune Response in Cows Exposed to Mycobacterium avium Subsp. paratuberculosis
- PMID: 29581962
- PMCID: PMC5822771
- DOI: 10.1155/2018/1450828
Protein Kinase G Induces an Immune Response in Cows Exposed to Mycobacterium avium Subsp. paratuberculosis
Abstract
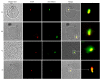
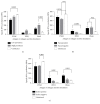
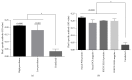
To establish infection, pathogens secrete virulence factors, such as protein kinases and phosphatases, to modulate the signal transduction pathways used by host cells to initiate immune response. The protein MAP3893c is annotated in the genome sequence of Mycobacterium avium subspecies paratuberculosis (MAP), the causative agent of Johne's disease, as the serine/threonine protein kinase G (PknG). In this work, we report that PknG is a functional kinase that is secreted within macrophages at early stages of infection. The antigen is able to induce an immune response from cattle exposed to MAP in the form of interferon gamma production after stimulation of whole blood with PknG. These findings suggest that PknG may contribute to the pathogenesis of MAP by phosphorylating macrophage signalling and/or adaptor molecules as observed with other pathogenic mycobacterial species.
Figures


References
-
- Johne’s disease on U.S. dairies, 1991-2007, (2008)
-
- Abubakar I., Myhill D., Aliyu S. H., Hunter P. R. Detection of Mycobacterium avium subspecies paratubercubsis from patients with Crohn's disease using nucleic acid-based techniques: a systematic review and meta-analysis. Inflammatory Bowel Diseases. 2008;14(3):401–410. doi: 10.1002/ibd.20276. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

