Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742)
- PMID: 29584545
- PMCID: PMC5941615
- DOI: 10.1200/JCO.2017.77.0388
Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742)
Erratum in
-
Erratum.J Clin Oncol. 2018 Aug 1;36(22):2360. doi: 10.1200/JCO.2018.79.3349. J Clin Oncol. 2018. PMID: 31329707 Free PMC article.
Abstract
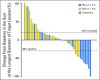
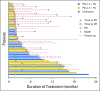
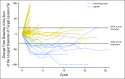
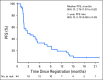
Purpose This multinational study evaluated the antitumor activity of nivolumab in nasopharyngeal carcinoma (NPC). Tumor and plasma-based biomarkers were investigated in an exploratory analysis. Patients and Methods Patients with multiply pretreated recurrent or metastatic NPC were treated with nivolumab until disease progression. The primary end point was objective response rate (ORR) and secondary end points included survival and toxicity. The expression of programmed death-ligand 1 (PD-L1) and human leukocyte antigens A and B in archived tumors and plasma clearance of Epstein-Barr virus DNA were correlated with ORR and survival. Results A total of 44 patients were evaluated and the overall ORR was 20.5% (complete response, n = 1; partial response, n = 8). Nine patients received nivolumab for > 12 months (20%). The 1-year overall survival rate was 59% (95% CI, 44.3% to 78.5%) and 1-year progression-free survival (PFS) rate was 19.3% (95% CI, 10.1% to 37.2%). There was no statistical correlation between ORR and the biomarkers; however, a descriptive analysis showed that the proportion of patients who responded was higher among those with PD-L1 positive tumors (> 1% expression) than those with PD-L1-negative tumors. The loss of expression of one or both human leukocyte antigen class 1 proteins was associated with better PFS than when both proteins were expressed (1-year PFS, 30.9% v 5.6%; log-rank P = .01). There was no association between survival and PD-L1 expression or plasma Epstein-Barr virus DNA clearance. There was no unexpected toxicity to nivolumab. Conclusion Nivolumab has promising activity in NPC and the 1-year overall survival rate compares favorably with historic data in similar populations. Additional evaluation in a randomized setting is warranted. The biomarker results were hypothesis generating and validation in larger cohorts is needed.
Trial registration: ClinicalTrials.gov NCT02339558.
Figures



References
-
- Lo YM, Leung SF, Chan LY, et al. : Kinetics of plasma Epstein-Barr virus DNA during radiation therapy for nasopharyngeal carcinoma. Cancer Res 60:2351-2355, 2000 - PubMed
-
- Lin DC, Meng X, Hazawa M, et al. : The genomic landscape of nasopharyngeal carcinoma. Nat Genet 46:866-871, 2014 - PubMed
-
- Muro K, Chung HC, Shankaran V, et al. : Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol 17:717-726, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

