Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial
- PMID: 29584546
- PMCID: PMC6075855
- DOI: 10.1200/JCO.2017.76.0793
Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial
Erratum in
-
Errata.J Clin Oncol. 2018 Sep 10;36(26):2748. doi: 10.1200/JCO.2018.79.3547. J Clin Oncol. 2018. PMID: 31329709 Free PMC article.
Abstract
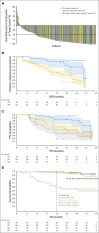
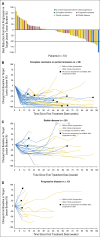
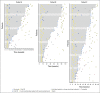
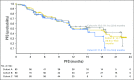
Purpose Genetic alterations causing overexpression of programmed death-1 ligands are near universal in classic Hodgkin lymphoma (cHL). Nivolumab, a programmed death-1 checkpoint inhibitor, demonstrated efficacy in relapsed/refractory cHL after autologous hematopoietic cell transplantation (auto-HCT) in initial analyses of one of three cohorts from the CheckMate 205 study of nivolumab for cHL. Here, we assess safety and efficacy after extended follow-up of all three cohorts. Methods This multicenter, single-arm, phase II study enrolled patients with relapsed/refractory cHL after auto-HCT treatment failure into cohorts by treatment history: brentuximab vedotin (BV)-naïve (cohort A), BV received after auto-HCT (cohort B), and BV received before and/or after auto-HCT (cohort C). All patients received nivolumab 3 mg/kg every 2 weeks until disease progression/unacceptable toxicity. The primary end point was objective response rate per independent radiology review committee. Results Overall, 243 patients were treated; 63 in cohort A, 80 in cohort B, and 100 in cohort C. After a median follow-up of 18 months, 40% continued to receive treatment. The objective response rate was 69% (95% CI, 63% to 75%) overall and 65% to 73% in each cohort. Overall, the median duration of response was 16.6 months (95% CI, 13.2 to 20.3 months), and median progression-free survival was 14.7 months (95% CI, 11.3 to 18.5 months). Of 70 patients treated past conventional disease progression, 61% of those evaluable had stable or further reduced target tumor burdens. The most common grade 3 to 4 drug-related adverse events were lipase increases (5%), neutropenia (3%), and ALT increases (3%). Twenty-nine deaths occurred; none were considered treatment related. Conclusion With extended follow-up, responses to nivolumab were frequent and durable. Nivolumab seems to be associated with a favorable safety profile and long-term benefits across a broad spectrum of patients with relapsed/refractory cHL.
Trial registration: ClinicalTrials.gov NCT02181738.
Figures



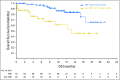
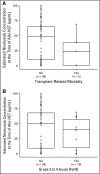
Comment in
-
Nivolumab effective in treatment-resistant HL.Nat Rev Clin Oncol. 2018 Jul;15(7):402. doi: 10.1038/s41571-018-0022-2. Nat Rev Clin Oncol. 2018. PMID: 29670243 No abstract available.
References
-
- von Tresckow B, Müller H, Eichenauer DA, et al. : Outcome and risk factors of patients with Hodgkin Lymphoma who relapse or progress after autologous stem cell transplant. Leuk Lymphoma 55:1922-1924, 2014 - PubMed
-
- Crump M: Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. Hematology Am Soc Hematol Educ Program 2008:326-333, 2008 - PubMed
-
- Bartlett NL, Niedzwiecki D, Johnson JL, et al. : Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin’s lymphoma: CALGB 59804. Ann Oncol 18:1071-1079, 2007 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

