Constitutively active follicle-stimulating hormone receptor enables androgen-independent spermatogenesis
- PMID: 29584617
- PMCID: PMC5919831
- DOI: 10.1172/JCI96794
Constitutively active follicle-stimulating hormone receptor enables androgen-independent spermatogenesis
Abstract
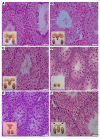
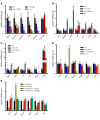
Spermatogenesis is regulated by the 2 pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). This process is considered impossible without the absolute requirement of LH-stimulated testicular testosterone (T) production. The role of FSH remains unclear because men and mice with inactivating FSH receptor (FSHR) mutations are fertile. We revisited the role of FSH in spermatogenesis using transgenic mice expressing a constitutively strongly active FSHR mutant in a LH receptor-null (LHR-null) background. The mutant FSHR reversed the azoospermia and partially restored fertility of Lhr-/- mice. The finding was initially ascribed to the residual Leydig cell T production. However, when T action was completely blocked with the potent antiandrogen flutamide, spermatogenesis persisted. Hence, completely T-independent spermatogenesis is possible through strong FSHR activation, and the dogma of T being a sine qua non for spermatogenesis may need modification. The mechanism for the finding appeared to be that FSHR activation maintained the expression of Sertoli cell genes considered androgen dependent. The translational message of our findings is the possibility of developing a new strategy of high-dose FSH treatment for spermatogenic failure. Our findings also provide an explanation of molecular pathogenesis for Pasqualini syndrome (fertile eunuchs; LH/T deficiency with persistent spermatogenesis) and explain how the hormonal regulation of spermatogenesis has shifted from FSH to T dominance during evolution.
Keywords: Endocrinology; Fertility; Reproductive Biology; Reproductive biochemistry.
Conflict of interest statement
Figures

References
-
- Smith LB, Walker WH. Hormonal signaling in the testis. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction. Vol. 1. Waltham, Massachusetts, USA: Academic Press; 2015;637–675.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

