Role of proNGF/p75 signaling in bladder dysfunction after spinal cord injury
- PMID: 29584618
- PMCID: PMC5919823
- DOI: 10.1172/JCI97837
Role of proNGF/p75 signaling in bladder dysfunction after spinal cord injury
Abstract
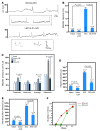
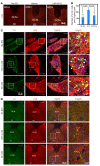
Loss of bladder control is a challenging outcome facing patients with spinal cord injury (SCI). We report that systemic blocking of pro-nerve growth factor (proNGF) signaling through p75 with a CNS-penetrating small-molecule p75 inhibitor resulted in significant improvement in bladder function after SCI in rodents. The usual hyperreflexia was attenuated with normal bladder pressure, and automatic micturition was acquired weeks earlier than in the controls. The improvement was associated with increased excitatory input to the spinal cord, in particular onto the tyrosine hydroxylase-positive fibers in the dorsal commissure. The drug also had an effect on the bladder itself, as the urothelial hyperplasia and detrusor hypertrophy that accompany SCI were largely prevented. Urothelial cell loss that precedes hyperplasia was dependent on p75 in response to urinary proNGF that is detected after SCI in rodents and humans. Surprisingly, death of urothelial cells and the ensuing hyperplastic response were beneficial to functional recovery. Deleting p75 from the urothelium prevented urothelial death, but resulted in reduction in overall voiding efficiency after SCI. These results unveil a dual role of proNGF/p75 signaling in bladder function under pathological conditions with a CNS effect overriding the peripheral one.
Keywords: Neuroscience; Urology.
Conflict of interest statement
Figures


References
-
- Ochodnický P, Cruz CD, Yoshimura N, Michel MC. Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target. Neurourol Urodyn. 2011;30(7):1227–1241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

