PAI1 mediates fibroblast-mast cell interactions in skin fibrosis
- PMID: 29584619
- PMCID: PMC5919880
- DOI: 10.1172/JCI99088
PAI1 mediates fibroblast-mast cell interactions in skin fibrosis
Abstract
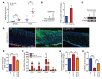
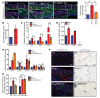
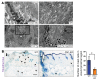
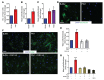
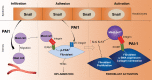
Fibrosis is a prevalent pathological condition arising from the chronic activation of fibroblasts. This activation results from the extensive intercellular crosstalk mediated by both soluble factors and direct cell-cell connections. Prominent among these are the interactions of fibroblasts with immune cells, in which the fibroblast-mast cell connection, although acknowledged, is relatively unexplored. We have used a Tg mouse model of skin fibrosis, based on expression of the transcription factor Snail in the epidermis, to probe the mechanisms regulating mast cell activity and the contribution of these cells to this pathology. We have discovered that Snail-expressing keratinocytes secrete plasminogen activator inhibitor type 1 (PAI1), which functions as a chemotactic factor to increase mast cell infiltration into the skin. Moreover, we have determined that PAI1 upregulates intercellular adhesion molecule type 1 (ICAM1) expression on dermal fibroblasts, rendering them competent to bind to mast cells. This heterotypic cell-cell adhesion, also observed in the skin fibrotic disorder scleroderma, culminates in the reciprocal activation of both mast cells and fibroblasts, leading to the cascade of events that promote fibrogenesis. Thus, we have identified roles for PAI1 in the multifactorial program of fibrogenesis that expand its functional repertoire beyond its canonical role in plasmin-dependent processes.
Keywords: Cell Biology; Fibrosis; Inflammation; Innate immunity; Skin.
Conflict of interest statement
Figures
Similar articles
-
Connective tissue growth factor causes persistent proalpha2(I) collagen gene expression induced by transforming growth factor-beta in a mouse fibrosis model.J Cell Physiol. 2005 May;203(2):447-56. doi: 10.1002/jcp.20251. J Cell Physiol. 2005. PMID: 15605379
-
Role of stem cell factor and monocyte chemoattractant protein-1 in the interaction between fibroblasts and mast cells in fibrosis.J Dermatol Sci. 2001 Jun;26(2):106-11. doi: 10.1016/s0923-1811(00)00164-x. J Dermatol Sci. 2001. PMID: 11378326
-
The production of collagen and the activity of mast-cell chymase increase in human skin after irradiation therapy.Exp Dermatol. 2004 Jun;13(6):364-71. doi: 10.1111/j.0906-6705.2004.00164.x. Exp Dermatol. 2004. PMID: 15186323
-
A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis.Int J Mol Sci. 2020 Dec 18;21(24):9673. doi: 10.3390/ijms21249673. Int J Mol Sci. 2020. PMID: 33353063 Free PMC article. Review.
-
Mechanisms of phototoxicity in porphyria cutanea tarda and erythropoietic protoporphyria.Immunol Ser. 1989;46:671-85. Immunol Ser. 1989. PMID: 2488874 Review.
Cited by
-
Tumor Cell Derived Lnc-FSD2-31:1 Contributes to Cancer-Associated Fibroblasts Activation in Pancreatic Ductal Adenocarcinoma Progression through Extracellular Vesicles Cargo MiR-4736.Adv Sci (Weinh). 2023 Apr;10(10):e2203324. doi: 10.1002/advs.202203324. Epub 2023 Feb 2. Adv Sci (Weinh). 2023. PMID: 36727832 Free PMC article.
-
Fibroblast activation in response to TGFβ1 is modulated by co-culture with endothelial cells in a vascular organ-on-chip platform.Front Mol Biosci. 2023 Jul 28;10:1160851. doi: 10.3389/fmolb.2023.1160851. eCollection 2023. Front Mol Biosci. 2023. PMID: 37577751 Free PMC article.
-
Intermittent hypoxia mediated by TSP1 dependent on STAT3 induces cardiac fibroblast activation and cardiac fibrosis.Elife. 2020 Jan 14;9:e49923. doi: 10.7554/eLife.49923. Elife. 2020. PMID: 31934850 Free PMC article.
-
Unraveling SSc Pathophysiology; The Myofibroblast.Front Immunol. 2018 Nov 13;9:2452. doi: 10.3389/fimmu.2018.02452. eCollection 2018. Front Immunol. 2018. PMID: 30483246 Free PMC article. Review.
-
Mast Cells Are Mediators of Fibrosis and Effector Cell Recruitment in Dermal Chronic Graft-vs.-Host Disease.Front Immunol. 2019 Oct 18;10:2470. doi: 10.3389/fimmu.2019.02470. eCollection 2019. Front Immunol. 2019. PMID: 31681336 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

