Blocking fatty acid-fueled mROS production within macrophages alleviates acute gouty inflammation
- PMID: 29584621
- PMCID: PMC5919807
- DOI: 10.1172/JCI94584
Blocking fatty acid-fueled mROS production within macrophages alleviates acute gouty inflammation
Abstract
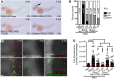
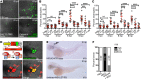
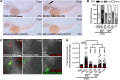
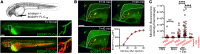
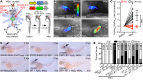
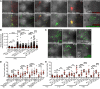
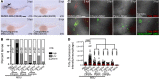
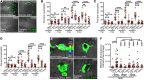
Gout is the most common inflammatory arthritis affecting men. Acute gouty inflammation is triggered by monosodium urate (MSU) crystal deposition in and around joints that activates macrophages into a proinflammatory state, resulting in neutrophil recruitment. A complete understanding of how MSU crystals activate macrophages in vivo has been difficult because of limitations of live imaging this process in traditional animal models. By live imaging the macrophage and neutrophil response to MSU crystals within an intact host (larval zebrafish), we reveal that macrophage activation requires mitochondrial ROS (mROS) generated through fatty acid oxidation. This mitochondrial source of ROS contributes to NF-κB-driven production of IL-1β and TNF-α, which promote neutrophil recruitment. We demonstrate the therapeutic utility of this discovery by showing that this mechanism is conserved in human macrophages and, via pharmacologic blockade, that it contributes to neutrophil recruitment in a mouse model of acute gouty inflammation. To our knowledge, this study is the first to uncover an immunometabolic mechanism of macrophage activation that operates during acute gouty inflammation. Targeting this pathway holds promise in the management of gout and, potentially, other macrophage-driven diseases.
Keywords: Arthritis; Immunology; Inflammation; Innate immunity; Macrophages.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

