Co-Culture with Human Osteoblasts and Exposure to Extremely Low Frequency Pulsed Electromagnetic Fields Improve Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells
- PMID: 29584629
- PMCID: PMC5979428
- DOI: 10.3390/ijms19040994
Co-Culture with Human Osteoblasts and Exposure to Extremely Low Frequency Pulsed Electromagnetic Fields Improve Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells
Abstract
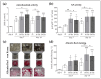
Human adipose-derived mesenchymal stem cells (Ad-MSCs) have been proposed as suitable option for cell-based therapies to support bone regeneration. In the bone environment, Ad-MSCs will receive stimuli from resident cells that may favor their osteogenic differentiation. There is recent evidence that this process can be further improved by extremely low frequency pulsed electromagnetic fields (ELF-PEMFs). Thus, the project aimed at (i) investigating whether co-culture conditions of human osteoblasts (OBs) and Ad-MSCs have an impact on their proliferation and osteogenic differentiation; (ii) whether this effect can be further improved by repetitive exposure to two specific ELF-PEMFs (16 and 26 Hz); (iii) and the effect of these ELF-PEMFs on human osteoclasts (OCs). Osteogenic differentiation was improved by co-culturing OBs and Ad-MSCs when compared to the individual mono-cultures. An OB to Ad-MSC ratio of 3:1 had best effects on total protein content, alkaline phosphatase (AP) activity, and matrix mineralization. Osteogenic differentiation was further improved by both ELF-PEMFs investigated. Interestingly, only repetitive exposure to 26 Hz ELF-PEMF increased Trap5B activity in OCs. Considering this result, a treatment with gradually increasing frequency might be of interest, as the lower frequency (16 Hz) could enhance bone formation, while the higher frequency (26 Hz) could enhance bone remodeling.
Keywords: extremely low frequency pulsed electromagnetic fields (ELF-PEMF); primary human adipose-derived mesenchymal stem cells (Ad-MSCs); primary human osteoblasts (OBs); primary human osteoclasts (OCs).
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures




Similar articles
-
Pulsed electromagnetic fields stimulate osteogenic differentiation in human bone marrow and adipose tissue derived mesenchymal stem cells.Bioelectromagnetics. 2014 Sep;35(6):426-36. doi: 10.1002/bem.21862. Epub 2014 Aug 6. Bioelectromagnetics. 2014. PMID: 25099126
-
Pulsed magnetic therapy increases osteogenic differentiation of mesenchymal stem cells only if they are pre-committed.Life Sci. 2016 May 1;152:44-51. doi: 10.1016/j.lfs.2016.03.020. Epub 2016 Mar 12. Life Sci. 2016. PMID: 26979772
-
Donor Site Location Is Critical for Proliferation, Stem Cell Capacity, and Osteogenic Differentiation of Adipose Mesenchymal Stem/Stromal Cells: Implications for Bone Tissue Engineering.Int J Mol Sci. 2018 Jun 26;19(7):1868. doi: 10.3390/ijms19071868. Int J Mol Sci. 2018. PMID: 29949865 Free PMC article.
-
The cellular effects of Pulsed Electromagnetic Fields on osteoblasts: A review.Bioelectromagnetics. 2019 May;40(4):211-233. doi: 10.1002/bem.22187. Epub 2019 Mar 25. Bioelectromagnetics. 2019. PMID: 30908726 Review.
-
Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications.Int J Mol Sci. 2021 Jan 15;22(2):809. doi: 10.3390/ijms22020809. Int J Mol Sci. 2021. PMID: 33467447 Free PMC article. Review.
Cited by
-
Asperosaponin VI stimulates osteogenic differentiation of rat adipose-derived stem cells.Regen Ther. 2019 May 10;11:17-24. doi: 10.1016/j.reth.2019.03.007. eCollection 2019 Dec. Regen Ther. 2019. PMID: 31193169 Free PMC article.
-
Direct Current Electrical Fields Improve Experimental Wound Healing by Activation of Cytokine Secretion and Erk1/2 Pathway Stimulation.Life (Basel). 2021 Nov 5;11(11):1195. doi: 10.3390/life11111195. Life (Basel). 2021. PMID: 34833071 Free PMC article.
-
Pulsating electromagnetic fields for perineal lacerations and surgical wounds healing in the postpartum: a pilot study.Arch Gynecol Obstet. 2024 Oct;310(4):1997-2006. doi: 10.1007/s00404-024-07671-3. Epub 2024 Aug 21. Arch Gynecol Obstet. 2024. PMID: 39164504 Free PMC article.
-
Establishment of a human 3D in vitro liver-bone model as a potential system for drug toxicity screening.Arch Toxicol. 2025 Jan;99(1):333-356. doi: 10.1007/s00204-024-03899-9. Epub 2024 Nov 6. Arch Toxicol. 2025. PMID: 39503877 Free PMC article.
-
Modulation of Macrophage Activity by Pulsed Electromagnetic Fields in the Context of Fracture Healing.Bioengineering (Basel). 2021 Oct 29;8(11):167. doi: 10.3390/bioengineering8110167. Bioengineering (Basel). 2021. PMID: 34821733 Free PMC article.
References
-
- Dominici M., le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

