Inhibition of Zika Virus Replication by Silvestrol
- PMID: 29584632
- PMCID: PMC5923443
- DOI: 10.3390/v10040149
Inhibition of Zika Virus Replication by Silvestrol
Abstract
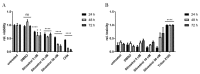
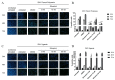
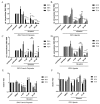
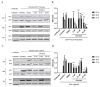
The Zika virus (ZIKV) outbreak in 2016 in South America with specific pathogenic outcomes highlighted the need for new antiviral substances with broad-spectrum activities to react quickly to unexpected outbreaks of emerging viral pathogens. Very recently, the natural compound silvestrol isolated from the plant Aglaia foveolata was found to have very potent antiviral effects against the (-)-strand RNA-virus Ebola virus as well as against Corona- and Picornaviruses with a (+)-strand RNA-genome. This antiviral activity is based on the impaired translation of viral RNA by the inhibition of the DEAD-box RNA helicase eukaryotic initiation factor-4A (eIF4A) which is required to unwind structured 5´-untranslated regions (5'-UTRs) of several proto-oncogenes and thereby facilitate their translation. Zika virus is a flavivirus with a positive-stranded RNA-genome harboring a 5'-capped UTR with distinct secondary structure elements. Therefore, we investigated the effects of silvestrol on ZIKV replication in A549 cells and primary human hepatocytes. Two different ZIKV strains were used. In both infected A549 cells and primary human hepatocytes, silvestrol has the potential to exert a significant inhibition of ZIKV replication for both analyzed strains, even though the ancestor strain from Uganda is less sensitive to silvestrol. Our data might contribute to identify host factors involved in the control of ZIKV infection and help to develop antiviral concepts that can be used to treat a variety of viral infections without the risk of resistances because a host protein is targeted.
Keywords: Ziks virus; antiviral; eIF4A; hepatocytes; silvestrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Zhu Z., Chan J.F.-W., Tee K.-M., Choi G.K.-Y., Lau S.K.-P., Woo P.C.-Y., Tse H., Yuen K.-Y. Comparative genomic analysis of pre-epidemic and epidemic Zika virus strains for virological factors potentially associated with the rapidly expanding epidemic. Emerg. Microbes Infect. 2016;5:e22. doi: 10.1038/emi.2016.48. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

