Charge-Controlled Synthetic Hyaluronan-Based Cell Matrices
- PMID: 29584672
- PMCID: PMC6017843
- DOI: 10.3390/molecules23040769
Charge-Controlled Synthetic Hyaluronan-Based Cell Matrices
Abstract
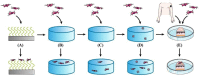
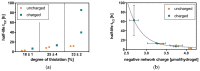
The extracellular matrix (ECM) represents a highly charged and hydrated network in which different cells in vertebrate tissues are embedded. Hydrogels as minimal ECM mimetics with a controlled chemistry offer the opportunity to vary material properties by varying the negative network charge. In this paper, a synthetic biology model of the ECM based on natural and highly negatively charged polyelectrolyte hyaluronic acid (HA) is characterized with specific emphasis on its charge-related bioactivity. Therefore, the thiol-Michael addition click reaction is used to produce HA hydrogels with defined network structure and charge density. The presented hydrogels show enzymatic degradability and cell attachment. These properties depend on both covalent and electrostatic interactions within the hydrogel network. Furthermore, no unspecific or specific attachment of proteins to the presented hydrogels is observed. In addition, these fundamental insights into charge-related ECM behavior and the influence of electrostatic properties could also lead to innovations in existing biomedical products.
Keywords: cell attachment; enzymatic degradation; glycosaminoglycans; hyaluronan; polyelectrolyte hydrogel; synthetic ECM; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Alberts B. Molecular Biology of the Cell. 5th ed. Garland; New York, NY, USA: 2007. pp. 1–15.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

