Particulate Guanylyl Cyclase A/cGMP Signaling Pathway in the Kidney: Physiologic and Therapeutic Indications
- PMID: 29584705
- PMCID: PMC5979439
- DOI: 10.3390/ijms19041006
Particulate Guanylyl Cyclase A/cGMP Signaling Pathway in the Kidney: Physiologic and Therapeutic Indications
Abstract
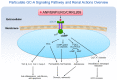
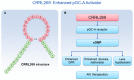
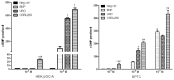
The particulate guanylyl cyclase A (pGC-A)/cGMP pathway plays important roles in regulating renal physiological function and as well as in counteracting pathophysiological conditions. Naturally occurring peptide pGC-A activators consist of atrial natriuretic peptide (ANP), b-type NP (BNP), and urodilatin (URO). These activators bind and activate pGC-A, generating the second messenger cyclic 3',5' guanosine monophosphate (cGMP). Cyclic GMP binds to downstream pathway effector molecules including protein kinase G (PKG), cGMP-gated ion channels, and phosphodiesterases (PDEs). These mediators result in a variety of physiological actions in the kidney, including diuresis, natriuresis, increased glomerular filtration rate (GFR) and organ protection, thus, opposing renal cellular injury and remodeling. Downstream proteins regulated by PKG include collagen 1 (Col-1), transforming growth factor beta (TGF-β) and apoptosis-related proteins. In addition to their physiological regulatory effects, pGC-A/cGMP signaling is critical for preserving renal homeostasis in different renal diseases such as acute kidney injury (AKI). Regarding therapeutic options, native pGC-A activators have short half-lives and their activity can be further enhanced by advances in innovative peptide engineering. Thus, novel designer peptide pGC-A activators with enhanced renal activity are under development.
Keywords: acute kidney injury; cGMP; natriuretic peptide; particulate guanylyl cyclase A; protein kinase G; renal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cenderitide: structural requirements for the creation of a novel dual particulate guanylyl cyclase receptor agonist with renal-enhancing in vivo and ex vivo actions.Eur Heart J Cardiovasc Pharmacother. 2016 Apr;2(2):98-105. doi: 10.1093/ehjcvp/pvv040. Epub 2015 Dec 10. Eur Heart J Cardiovasc Pharmacother. 2016. PMID: 27340557 Free PMC article.
-
CRRL269: a novel designer and renal-enhancing pGC-A peptide activator.Am J Physiol Regul Integr Comp Physiol. 2018 Mar 1;314(3):R407-R414. doi: 10.1152/ajpregu.00286.2017. Epub 2017 Nov 29. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29187381 Free PMC article.
-
C53: A novel particulate guanylyl cyclase B receptor activator that has sustained activity in vivo with anti-fibrotic actions in human cardiac and renal fibroblasts.J Mol Cell Cardiol. 2019 May;130:140-150. doi: 10.1016/j.yjmcc.2019.03.024. Epub 2019 Apr 4. J Mol Cell Cardiol. 2019. PMID: 30954448 Free PMC article.
-
The cGMP system: components and function.Biol Chem. 2020 Mar 26;401(4):447-469. doi: 10.1515/hsz-2019-0386. Biol Chem. 2020. PMID: 31747372 Review.
-
The guanylyl cyclase receptor family.New Biol. 1990 Jun;2(6):499-504. New Biol. 1990. PMID: 1982420 Review.
Cited by
-
BAY-7081: A Potent, Selective, and Orally Bioavailable Cyanopyridone-Based PDE9A Inhibitor.J Med Chem. 2022 Dec 22;65(24):16420-16431. doi: 10.1021/acs.jmedchem.2c01267. Epub 2022 Dec 7. J Med Chem. 2022. PMID: 36475653 Free PMC article.
-
Localization of natriuretic peptide receptors A, B, and C in healthy and diseased mouse kidneys.Pflugers Arch. 2023 Mar;475(3):343-360. doi: 10.1007/s00424-022-02774-9. Epub 2022 Dec 8. Pflugers Arch. 2023. PMID: 36480070 Free PMC article.
-
Carbon monoxide signaling and soluble guanylyl cyclase: Facts, myths, and intriguing possibilities.Biochem Pharmacol. 2022 Jun;200:115041. doi: 10.1016/j.bcp.2022.115041. Epub 2022 Apr 18. Biochem Pharmacol. 2022. PMID: 35447132 Free PMC article. Review.
-
Kidney dopamine D1-like receptors and angiotensin 1-7 interaction inhibits renal Na+ transporters.Am J Physiol Renal Physiol. 2019 Oct 1;317(4):F949-F956. doi: 10.1152/ajprenal.00135.2019. Epub 2019 Aug 14. Am J Physiol Renal Physiol. 2019. PMID: 31411069 Free PMC article.
-
Hormonal Regulation of Renal Fibrosis.Life (Basel). 2022 May 16;12(5):737. doi: 10.3390/life12050737. Life (Basel). 2022. PMID: 35629404 Free PMC article. Review.
References
-
- Kellum J.A., Lameire N., Aspelin P., Barsoum R.S., Burdmann E.A., Goldstein S.L., Herzog C.A., Joannidis M., Kribben A., Levey A.S. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012;2:1–138.
-
- Hoste E.A., Bagshaw S.M., Bellomo R., Cely C.M., Colman R., Cruz D.N., Edipidis K., Forni L.G., Gomersall C.D., Govil D., et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

