Evaluation of e-liquid toxicity using an open-source high-throughput screening assay
- PMID: 29584716
- PMCID: PMC5870948
- DOI: 10.1371/journal.pbio.2003904
Evaluation of e-liquid toxicity using an open-source high-throughput screening assay
Abstract
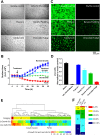
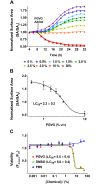
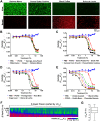
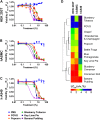
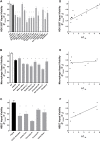
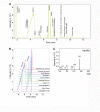
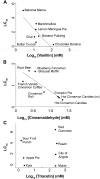
The e-liquids used in electronic cigarettes (E-cigs) consist of propylene glycol (PG), vegetable glycerin (VG), nicotine, and chemical additives for flavoring. There are currently over 7,700 e-liquid flavors available, and while some have been tested for toxicity in the laboratory, most have not. Here, we developed a 3-phase, 384-well, plate-based, high-throughput screening (HTS) assay to rapidly triage and validate the toxicity of multiple e-liquids. Our data demonstrated that the PG/VG vehicle adversely affected cell viability and that a large number of e-liquids were more toxic than PG/VG. We also performed gas chromatography-mass spectrometry (GC-MS) analysis on all tested e-liquids. Subsequent nonmetric multidimensional scaling (NMDS) analysis revealed that e-liquids are an extremely heterogeneous group. Furthermore, these data indicated that (i) the more chemicals contained in an e-liquid, the more toxic it was likely to be and (ii) the presence of vanillin was associated with higher toxicity values. Further analysis of common constituents by electron ionization revealed that the concentration of cinnamaldehyde and vanillin, but not triacetin, correlated with toxicity. We have also developed a publicly available searchable website (www.eliquidinfo.org). Given the large numbers of available e-liquids, this website will serve as a resource to facilitate dissemination of this information. Our data suggest that an HTS approach to evaluate the toxicity of multiple e-liquids is feasible. Such an approach may serve as a roadmap to enable bodies such as the Food and Drug Administration (FDA) to better regulate e-liquid composition.
Conflict of interest statement
The authors have declared that no competing interests exist. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Figures

References
-
- Kim KH, Kabir E, Jahan SA. Review of electronic cigarettes as tobacco cigarette substitutes: Their potential human health impact. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2016;34(4):262–75. doi: 10.1080/10590501.2016.1236604 . - DOI - PubMed
-
- Geiser M, Kreyling WG. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol. 2010;7:2 doi: 10.1186/1743-8977-7-2 . - DOI - PMC - PubMed
-
- Chen J, Bullen C, Dirks K. A Comparative Health Risk Assessment of Electronic Cigarettes and Conventional Cigarettes. Int J Environ Res Public Health. 2017;14(4). doi: 10.3390/ijerph14040382 . - DOI - PMC - PubMed
-
- McNeill A BL, Calder R, Hitchman SC, Hajek P, McRobbie H. E-cigarettes: an evidence update. Public Health England. 2015.
-
- Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health. 2014;11(11):11192–200. doi: 10.3390/ijerph111111192 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

