Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques
- PMID: 29584730
- PMCID: PMC5870946
- DOI: 10.1371/journal.pmed.1002535
Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques
Abstract
Background: Despite repeated outbreaks, in particular the devastating 2014-2016 epidemic, there is no effective treatment validated for patients with Ebola virus disease (EVD). Among the drug candidates is the broad-spectrum polymerase inhibitor favipiravir, which showed a good tolerance profile in patients with EVD (JIKI trial) but did not demonstrate a strong antiviral efficacy. In order to gain new insights into the antiviral efficacy of favipiravir and improve preparedness and public health management of future outbreaks, we assess the efficacy achieved by ascending doses of favipiravir in Ebola-virus-infected nonhuman primates (NHPs).
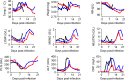
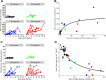
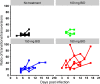
Methods and findings: A total of 26 animals (Macaca fascicularis) were challenged intramuscularly at day 0 with 1,000 focus-forming units of Ebola virus Gabon 2001 strain and followed for 21 days (study termination). This included 13 animals left untreated and 13 treated with doses of 100, 150, and 180 mg/kg (N = 3, 5, and 5, respectively) favipiravir administered intravenously twice a day for 14 days, starting 2 days before infection. All animals left untreated or treated with 100 mg/kg died within 10 days post-infection, while animals receiving 150 and 180 mg/kg had extended survival (P < 0.001 and 0.001, respectively, compared to untreated animals), leading to a survival rate of 40% (2/5) and 60% (3/5), respectively, at day 21. Favipiravir inhibited viral replication (molecular and infectious viral loads) in a drug-concentration-dependent manner (P values < 0.001), and genomic deep sequencing analyses showed an increase in virus mutagenesis over time. These results allowed us to identify that plasma trough favipiravir concentrations greater than 70-80 μg/ml were associated with reduced viral loads, lower virus infectivity, and extended survival. These levels are higher than those found in the JIKI trial, where patients had median trough drug concentrations equal to 46 and 26 μg/ml at day 2 and day 4 post-treatment, respectively, and suggest that the dosing regimen in the JIKI trial was suboptimal. The environment of a biosafety level 4 laboratory introduces a number of limitations, in particular the difficulty of conducting blind studies and performing detailed pharmacological assessments. Further, the extrapolation of the results to patients with EVD is limited by the fact that the model is fully lethal and that treatment initiation in patients with EVD is most often initiated several days after infection, when symptoms and high levels of viral replication are already present.
Conclusions: Our results suggest that favipiravir may be an effective antiviral drug against Ebola virus that relies on RNA chain termination and possibly error catastrophe. These results, together with previous data collected on tolerance and pharmacokinetics in both NHPs and humans, support a potential role for high doses of favipiravir for future human interventions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- World Health Organization. Situation report: Ebola virus disease. Geneva: World Health Organization; 2016. June 10 [cited 2018 Feb 22]. Available from: http://apps.who.int/iris/bitstream/10665/208883/1/ebolasitrep_10Jun2016_....
-
- Sissoko D, Laouenan C, Folkesson E, M’lebing A-B, Beavogui A-H, Baize S, et al. Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016;13:e1001967 doi: 10.1371/journal.pmed.1001967 - DOI - PMC - PubMed
-
- Dunning J, Kennedy SB, Antierens A, Whitehead J, Ciglenecki I, Carson G, et al. Experimental treatment of Ebola virus disease with brincidofovir. PLoS ONE. 2016;11:e0162199 doi: 10.1371/journal.pone.0162199 - DOI - PMC - PubMed
-
- PREVAIL II Writing Group, Multi-National PREVAIL II Study Team. A Randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med. 2016;375:1448–56. doi: 10.1056/NEJMoa1604330 - DOI - PMC - PubMed
-
- Dunning J, Sahr F, Rojek A, Gannon F, Carson G, Idriss B, et al. Experimental treatment of Ebola virus disease with TKM-130803: a single-arm phase 2 clinical trial. PLoS Med. 2016;13:e1001997 doi: 10.1371/journal.pmed.1001997 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

