Association of Placebo, Indomethacin, Ibuprofen, and Acetaminophen With Closure of Hemodynamically Significant Patent Ductus Arteriosus in Preterm Infants: A Systematic Review and Meta-analysis
- PMID: 29584842
- PMCID: PMC5885871
- DOI: 10.1001/jama.2018.1896
Association of Placebo, Indomethacin, Ibuprofen, and Acetaminophen With Closure of Hemodynamically Significant Patent Ductus Arteriosus in Preterm Infants: A Systematic Review and Meta-analysis
Abstract
Importance: Despite increasing emphasis on conservative management of patent ductus arteriosus (PDA) in preterm infants, different pharmacotherapeutic interventions are used to treat those developing a hemodynamically significant PDA.
Objectives: To estimate the relative likelihood of hemodynamically significant PDA closure with common pharmacotherapeutic interventions and to compare adverse event rates.
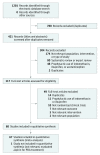
Data sources and study selection: The databases of MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched from inception until August 15, 2015, and updated on December 31, 2017, along with conference proceedings up to December 2017. Randomized clinical trials that enrolled preterm infants with a gestational age younger than 37 weeks treated with intravenous or oral indomethacin, ibuprofen, or acetaminophen vs each other, placebo, or no treatment for a clinically or echocardiographically diagnosed hemodynamically significant PDA.
Data extraction and synthesis: Data were independently extracted in pairs by 6 reviewers and synthesized with Bayesian random-effects network meta-analyses.
Main outcomes and measures: Primary outcome: hemodynamically significant PDA closure; secondary: included surgical closure, mortality, necrotizing enterocolitis, and intraventricular hemorrhage.
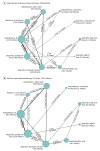
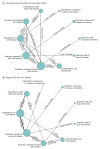
Results: In 68 randomized clinical trials of 4802 infants, 14 different variations of indomethacin, ibuprofen, or acetaminophen were used as treatment modalities. The overall PDA closure rate was 67.4% (2867 of 4256 infants). A high dose of oral ibuprofen was associated with a significantly higher odds of PDA closure vs a standard dose of intravenous ibuprofen (odds ratio [OR], 3.59; 95% credible interval [CrI], 1.64-8.17; absolute risk difference, 199 [95% CrI, 95-258] more per 1000 infants) and a standard dose of intravenous indomethacin (OR, 2.35 [95% CrI, 1.08-5.31]; absolute risk difference, 124 [95% CrI, 14-188] more per 1000 infants). Based on the ranking statistics, a high dose of oral ibuprofen ranked as the best pharmacotherapeutic option for PDA closure (mean surface under the cumulative ranking [SUCRA] curve, 0.89 [SD, 0.12]) and to prevent surgical PDA ligation (mean SUCRA, 0.98 [SD, 0.08]). There was no significant difference in the odds of mortality, necrotizing enterocolitis, or intraventricular hemorrhage with use of placebo or no treatment compared with any of the other treatment modalities.
Conclusions and relevance: A high dose of oral ibuprofen was associated with a higher likelihood of hemodynamically significant PDA closure vs standard doses of intravenous ibuprofen or intravenous indomethacin; placebo or no treatment did not significantly change the likelihood of mortality, necrotizing enterocolitis, or intraventricular hemorrhage.
Trial registration: PROSPERO Identifier: CRD42015015797.
Conflict of interest statement
Figures







Comment in
-
What is the Most Efficacious Pharmacological Therapy for Patent Ductus Arteriosus Closure in Premature Infants?Ir Med J. 2018 Oct 11;111(9):816. Ir Med J. 2018. PMID: 30556665 No abstract available.
References
-
- Dollberg S, Lusky A, Reichman B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. J Pediatr Gastroenterol Nutr. 2005;40(2):184-188. - PubMed
-
- Brown ER. Increased risk of bronchopulmonary dysplasia in infants with patent ductus arteriosus. J Pediatr. 1979;95(5 pt 2):865-866. - PubMed
-
- Lipman B, Serwer GA, Brazy JE. Abnormal cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatrics. 1982;69(6):778-781. - PubMed
-
- Letshwiti JB, Semberova J, Pichova K, Dempsey EM, Franklin OM, Miletin J. A conservative treatment of patent ductus arteriosus in very low birth weight infants. Early Hum Dev. 2017;104:45-49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

