Sliding of a single lac repressor protein along DNA is tuned by DNA sequence and molecular switching
- PMID: 29584872
- PMCID: PMC6007606
- DOI: 10.1093/nar/gky208
Sliding of a single lac repressor protein along DNA is tuned by DNA sequence and molecular switching
Abstract
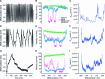
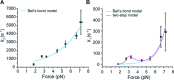
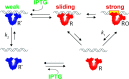
In any living cell, genome maintenance is carried out by DNA-binding proteins that recognize specific sequences among a vast amount of DNA. This includes fundamental processes such as DNA replication, DNA repair, and gene expression and regulation. Here, we study the mechanism of DNA target search by a single lac repressor protein (LacI) with ultrafast force-clamp spectroscopy, a sub-millisecond and few base-pair resolution technique based on laser tweezers. We measure 1D-diffusion of proteins on DNA at physiological salt concentrations with 20 bp resolution and find that sliding of LacI along DNA is sequence dependent. We show that only allosterically activated LacI slides along non-specific DNA sequences during target search, whereas the inhibited conformation does not support sliding and weakly interacts with DNA. Moreover, we find that LacI undergoes a load-dependent conformational change when it switches between sliding and strong binding to the target sequence. Our data reveal how DNA sequence and molecular switching regulate LacI target search process and provide a comprehensive model of facilitated diffusion for LacI.
Figures


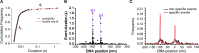


References
-
- Riggs A.D., Bourgeois S., Cohn M.. The lac represser-operator interaction. 3. Kinetic studies. J. Mol. Biol. 1970; 53:401–417. - PubMed
-
- Lewis M. The lac repressor. C. R. Biol. 2005; 1328:521–548. - PubMed
-
- Lewis M. Allostery and the lac operon. J. Mol. Biol. 2013; 425:2309–2316. - PubMed
-
- Lewis M., Chang G., Horton N.C., Kercher M.A., Pace H.C., Schumacher M.A., Brennan R.G., Lu P.. Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science. 1996; 271:1247–1254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

