Functional Characterization of Novel Atrial Fibrillation-Linked GJA5 (Cx40) Mutants
- PMID: 29587382
- PMCID: PMC5979441
- DOI: 10.3390/ijms19040977
Functional Characterization of Novel Atrial Fibrillation-Linked GJA5 (Cx40) Mutants
Abstract
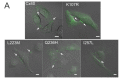
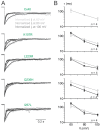
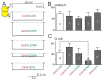
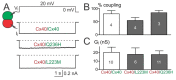
Atrial fibrillation (AF) is the most common form of cardiac arrhythmia. Recently, four novel heterozygous Cx40 mutations-K107R, L223M, Q236H, and I257L-were identified in 4 of 310 unrelated AF patients and a followup genetic analysis of the mutant carriers' families showed that the mutants were present in all the affected members. To study possible alterations associated with these Cx40 mutants, including their cellular localization and gap junction (GJ) function, we expressed GFP-tagged and untagged mutants in connexin-deficient model cells. All four Cx40 mutants showed clustered localization at cell-cell junctions similar to that observed of wildtype Cx40. However, cell pairs expressing Cx40 Q236H, but not the other individual mutants, displayed a significantly lower GJ coupling conductance (Gj) than wildtype Cx40. Similarly, co-expression of Cx40 Q236H with Cx43 resulted in a significantly lower Gj. Transjunctional voltage-dependent gating (Vj gating) properties were also altered in the GJs formed by Q236H. Reduced GJ function and altered Vj gating may play a role in promoting the Q236H carriers to AF.
Keywords: Vj gating; atrial fibrillation; connexin40; gap junction channel; patch clamp.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Functional formation of heterotypic gap junction channels by connexins-40 and -43.Channels (Austin). 2014;8(5):433-43. doi: 10.4161/19336950.2014.949188. Channels (Austin). 2014. PMID: 25483586 Free PMC article.
-
Atrial fibrillation-linked GJA5/connexin40 mutants impaired gap junctions via different mechanisms.FEBS Lett. 2014 Apr 17;588(8):1238-43. doi: 10.1016/j.febslet.2014.02.064. Epub 2014 Mar 19. FEBS Lett. 2014. PMID: 24656738 Review.
-
Atrial fibrillation-linked germline GJA5/connexin40 mutants showed an increased hemichannel function.PLoS One. 2014 Apr 14;9(4):e95125. doi: 10.1371/journal.pone.0095125. eCollection 2014. PLoS One. 2014. PMID: 24733048 Free PMC article.
-
The Connexin40A96S mutation from a patient with atrial fibrillation causes decreased atrial conduction velocities and sustained episodes of induced atrial fibrillation in mice.J Mol Cell Cardiol. 2013 Dec;65:19-32. doi: 10.1016/j.yjmcc.2013.09.008. Epub 2013 Sep 21. J Mol Cell Cardiol. 2013. PMID: 24060583
-
The role of connexin40 in atrial fibrillation.Cardiovasc Res. 2009 Oct 1;84(1):15-23. doi: 10.1093/cvr/cvp203. Epub 2009 Jun 17. Cardiovasc Res. 2009. PMID: 19535379 Review.
Cited by
-
Atrial Fibrillation: Focus on Myocardial Connexins and Gap Junctions.Biology (Basel). 2022 Mar 23;11(4):489. doi: 10.3390/biology11040489. Biology (Basel). 2022. PMID: 35453689 Free PMC article. Review.
-
Cytoskeletal Protein Variants Driving Atrial Fibrillation: Potential Mechanisms of Action.Cells. 2022 Jan 25;11(3):416. doi: 10.3390/cells11030416. Cells. 2022. PMID: 35159226 Free PMC article. Review.
-
Genetics and Pharmacogenetics of Atrial Fibrillation: A Mechanistic Perspective.JACC Basic Transl Sci. 2024 Feb 28;9(7):918-934. doi: 10.1016/j.jacbts.2023.12.006. eCollection 2024 Jul. JACC Basic Transl Sci. 2024. PMID: 39170958 Free PMC article. Review.
-
Pathophysiology of Atrial Fibrillation and Approach to Therapy in Subjects Less than 60 Years Old.Int J Mol Sci. 2024 Jan 7;25(2):758. doi: 10.3390/ijms25020758. Int J Mol Sci. 2024. PMID: 38255832 Free PMC article. Review.
-
Discovery of TBX20 as a Novel Gene Underlying Atrial Fibrillation.Biology (Basel). 2023 Aug 30;12(9):1186. doi: 10.3390/biology12091186. Biology (Basel). 2023. PMID: 37759586 Free PMC article.
References
-
- Fuster V., Ryden L.E., Cannom D.S., Crijns H.J., Curtis A.B., Ellenbogen K.A., Halperin J.L., Kay G.N., Le Huezey J.Y., Lowe J.E., et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2011;57:e101–e198. - PubMed
-
- Go A.S., Hylek E.M., Phillips K.A., Chang Y., Henault L.E., Selby J.V., Singer D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

