Osteogenic Potential of Pre-Osteoblastic Cells on a Chitosan-graft-Polycaprolactone Copolymer
- PMID: 29587410
- PMCID: PMC5951336
- DOI: 10.3390/ma11040490
Osteogenic Potential of Pre-Osteoblastic Cells on a Chitosan-graft-Polycaprolactone Copolymer
Abstract
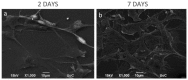
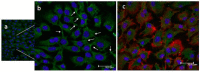
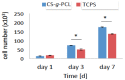
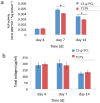
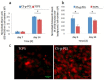
A chitosan-graft-polycaprolactone (CS-g-PCL) copolymer synthesized via a multi-step process was evaluated as a potential biomaterial for the adhesion and growth of MC3T3-E1 pre-osteoblastic cells. A strong adhesion of the MC3T3-E1 cells with a characteristic spindle-shaped morphology was observed from the first days of cell culture onto the copolymer surfaces. The viability and proliferation of the cells on the CS-g-PCL surfaces, after 3 and 7 days in culture, were significantly higher compared to the cells cultured on the tissue culture treated polystyrene (TCPS) control. The osteogenic potential of the pre-osteoblastic cells cultured on CS-g-PCL surfaces was evaluated by determining various osteogenic differentiation markers and was compared to the TCPS control surface. Specifically, alkaline phosphatase activity levels show significantly higher values at both time points compared to TCPS, while secreted collagen into the extracellular matrix was found to be higher on day 7. Calcium biomineralization deposited into the matrix is significantly higher for the CS-g-PCL copolymer after 14 days in culture, while the levels of intracellular osteopontin were significantly higher on the CS-g-PCL surfaces compared to TCPS. The enhanced osteogenic response of the MC3T3-E1 pre-osteoblasts cultured on CS-g-PCL reveals that the copolymer underpins the cell functions towards bone tissue formation and is thus an attractive candidate for use in bone tissue engineering.
Keywords: biomaterial; bone tissue engineering; chitosan; osteogenic potential; polycaprolactone; pre-osteoblastic cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Stevens M.M. Biomaterials for bone tissue engineering. Mater. Today. 2008;11:18–25. doi: 10.1016/S1369-7021(08)70086-5. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

