Multifaced Roles of the αvβ3 Integrin in Ehlers-Danlos and Arterial Tortuosity Syndromes' Dermal Fibroblasts
- PMID: 29587413
- PMCID: PMC5979373
- DOI: 10.3390/ijms19040982
Multifaced Roles of the αvβ3 Integrin in Ehlers-Danlos and Arterial Tortuosity Syndromes' Dermal Fibroblasts
Abstract
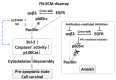
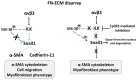
The αvβ3 integrin, an endothelial cells' receptor-binding fibronectin (FN) in the extracellular matrix (ECM) of blood vessels, regulates ECM remodeling during migration, invasion, angiogenesis, wound healing and inflammation, and is also involved in the epithelial mesenchymal transition. In vitro-grown human control fibroblasts organize a fibrillar network of FN, which is preferentially bound on the entire cell surface to its canonical α5β1 integrin receptor, whereas the αvβ3 integrin is present only in rare patches in focal contacts. We report on the preferential recruitment of the αvβ3 integrin, due to the lack of FN-ECM and its canonical integrin receptor, in dermal fibroblasts from Ehlers-Danlos syndromes (EDS) and arterial tortuosity syndrome (ATS), which are rare multisystem connective tissue disorders. We review our previous findings that unraveled different biological mechanisms elicited by the αvβ3 integrin in fibroblasts derived from patients affected with classical (cEDS), vascular (vEDS), hypermobile EDS (hEDS), hypermobility spectrum disorders (HSD), and ATS. In cEDS and vEDS, respectively, due to defective type V and type III collagens, αvβ3 rescues patients' fibroblasts from anoikis through a paxillin-p60Src-mediated cross-talk with the EGF receptor. In hEDS and HSD, without a defined molecular basis, the αvβ3 integrin transduces to the ILK-Snail1-axis inducing a fibroblast-to-myofibroblast-transition. In ATS cells, the deficiency of the dehydroascorbic acid transporter GLUT10 leads to redox imbalance, ECM disarray together with the activation of a non-canonical αvβ3 integrin-TGFBRII signaling, involving p125FAK/p60Src/p38MAPK. The characterization of these different biological functions triggered by αvβ3 provides insights into the multifaced nature of this integrin, at least in cultured dermal fibroblasts, offering future perspectives for research in this field.
Keywords: Ehlers–Danlos syndromes; apoptosis; arterial tortuosity syndrome; extracellular matrix; fibroblast-to-myofibroblast transition; fibronectin; αvβ3 integrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cellular and Molecular Mechanisms in the Pathogenesis of Classical, Vascular, and Hypermobile Ehlers‒Danlos Syndromes.Genes (Basel). 2019 Aug 12;10(8):609. doi: 10.3390/genes10080609. Genes (Basel). 2019. PMID: 31409039 Free PMC article. Review.
-
Dermal fibroblast-to-myofibroblast transition sustained by αvß3 integrin-ILK-Snail1/Slug signaling is a common feature for hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders.Biochim Biophys Acta Mol Basis Dis. 2018 Apr;1864(4 Pt A):1010-1023. doi: 10.1016/j.bbadis.2018.01.005. Epub 2018 Jan 5. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29309923
-
GLUT10 deficiency leads to oxidative stress and non-canonical αvβ3 integrin-mediated TGFβ signalling associated with extracellular matrix disarray in arterial tortuosity syndrome skin fibroblasts.Hum Mol Genet. 2015 Dec 1;24(23):6769-87. doi: 10.1093/hmg/ddv382. Epub 2015 Sep 16. Hum Mol Genet. 2015. PMID: 26376865 Free PMC article.
-
Human fibroblasts with mutations in COL5A1 and COL3A1 genes do not organize collagens and fibronectin in the extracellular matrix, down-regulate alpha2beta1 integrin, and recruit alphavbeta3 Instead of alpha5beta1 integrin.J Biol Chem. 2004 Apr 30;279(18):18157-68. doi: 10.1074/jbc.M312609200. Epub 2004 Feb 17. J Biol Chem. 2004. PMID: 14970208
-
Hypermobile Ehlers-Danlos syndromes: Complex phenotypes, challenging diagnoses, and poorly understood causes.Dev Dyn. 2021 Mar;250(3):318-344. doi: 10.1002/dvdy.220. Epub 2020 Aug 17. Dev Dyn. 2021. PMID: 32629534 Free PMC article. Review.
Cited by
-
Cellular and Molecular Mechanisms in the Pathogenesis of Classical, Vascular, and Hypermobile Ehlers‒Danlos Syndromes.Genes (Basel). 2019 Aug 12;10(8):609. doi: 10.3390/genes10080609. Genes (Basel). 2019. PMID: 31409039 Free PMC article. Review.
-
Type V Collagen in Scar Tissue Regulates the Size of Scar after Heart Injury.Cell. 2020 Aug 6;182(3):545-562.e23. doi: 10.1016/j.cell.2020.06.030. Epub 2020 Jul 3. Cell. 2020. PMID: 32621799 Free PMC article.
-
Modulating the extracellular matrix to treat wound healing defects in Ehlers-Danlos syndrome.iScience. 2024 Aug 6;27(9):110676. doi: 10.1016/j.isci.2024.110676. eCollection 2024 Sep 20. iScience. 2024. PMID: 39262784 Free PMC article.
-
Role of peptide-cell surface interactions in cosmetic peptide application.Front Pharmacol. 2023 Nov 13;14:1267765. doi: 10.3389/fphar.2023.1267765. eCollection 2023. Front Pharmacol. 2023. PMID: 38027006 Free PMC article.
-
Integrative Multi-Omics Approach in Vascular Ehlers-Danlos Syndrome: Further Insights into the Disease Mechanisms by Proteomic Analysis of Patient Dermal Fibroblasts.Biomedicines. 2024 Nov 30;12(12):2749. doi: 10.3390/biomedicines12122749. Biomedicines. 2024. PMID: 39767655 Free PMC article.
References
-
- Theocharis A.D., Gialeli C., Hascall V.C., Karamanos N.K. Extracellular matrix: A functional scaffold. In: Karamanos N.K., editor. Extracellular Matrix: Pathobiology and Signaling. Walter de Gruyter GmbH & Co. KG; Berlin, Germany: Boston, MA, USA: 2012. pp. 3–20.
-
- Karsdal M.A., Nielsen M.J., Sand J.M., Henriksen K., Genovese F., Bay-Jensen A.C., Smith V., Adamkewicz J.I., Christiansen C., Leeming D.J. Extracellular matrix remodeling: The common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev. Technol. 2013;11:70–92. doi: 10.1089/adt.2012.474. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

