Photophysical and Photocatalytic Properties of BiSnSbO₆ under Visible Light Irradiation
- PMID: 29587420
- PMCID: PMC5951337
- DOI: 10.3390/ma11040491
Photophysical and Photocatalytic Properties of BiSnSbO₆ under Visible Light Irradiation
Abstract
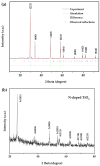
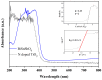
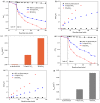
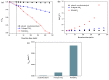
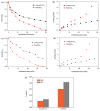
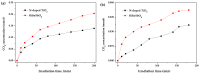
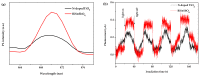
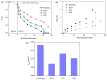
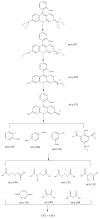
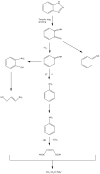
BiSnSbO₆ with strong photocatalytic activity was first fabricated by a high-temperature, solid-state sintering method. The resulting BiSnSbO₆ was characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), UV-vis diffuse reflectance spectroscopy (DRS) and X-ray photoelectron spectroscopy (XPS). The results showed that BiSnSbO₆, with a pyrochlore structure and a cubic crystal system by a space group Fd3m, was well crystallized. The lattice parameter or the band gap of BiSnSbO₆ was 10.234594 Å or 2.83 eV. Compared with N-doped TiO₂, BiSnSbO₆ showed higher photocatalytic activity in the degradation of benzotriazole and rhodamine B. The apparent first-order rate constant for BiSnSbO₆ in the degradation of benzotriazole and rhodamine B was 0.0182 min-1 and 0.0147 min-1, respectively. On the basis of the scavenger experiment, during the photocatalytic process, the main active species were arranged in order of increasing photodegradation rate: •OH < •O₂- < h⁺. The removal rate of benzotriazole or rhodamine B was approximately estimated to be 100% with BiSnSbO₆ as a photocatalyst after 200 min visible-light irradiation. Plentiful CO₂ produced by the experiment indicated that benzotriazole or rhodamine B was continuously mineralized during the photocatalytic process. Finally, the possible photodegradation pathways of benzotriazole and rhodamine B were deduced.
Keywords: BiSnSbO6; benzotriazole; photocatalytic degradation; rhodamine B; visible light irradiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Synthesis, Structural Property, Photophysical Property, Photocatalytic Property of Novel ZnBiErO₄ under Visible Light Irradiation.Materials (Basel). 2018 Feb 18;11(2):303. doi: 10.3390/ma11020303. Materials (Basel). 2018. PMID: 29463016 Free PMC article.
-
Synthesis, crystal structure, photodegradation kinetics and photocatalytic activity of novel photocatalyst ZnBiYO4.J Environ Sci (China). 2015 Mar 1;29:51-61. doi: 10.1016/j.jes.2014.06.051. Epub 2015 Jan 29. J Environ Sci (China). 2015. PMID: 25766013
-
Synthesis, Property Characterization and Photocatalytic Activity of the Polyaniline/BiYTi₂O₇ Polymer Composite.Polymers (Basel). 2017 Feb 23;9(3):69. doi: 10.3390/polym9030069. Polymers (Basel). 2017. PMID: 30970757 Free PMC article.
-
Investigation into the synthesis conditions of CuMoO4 by an in situ method and its photocatalytic properties under visible light irradiation.RSC Adv. 2020 Mar 6;10(16):9745-9759. doi: 10.1039/d0ra00496k. eCollection 2020 Mar 2. RSC Adv. 2020. PMID: 35497233 Free PMC article.
-
Investigation of the Photocatalytic Performance, Mechanism, and Degradation Pathways of Rhodamine B with Bi2O3 Microrods under Visible-Light Irradiation.Materials (Basel). 2024 Feb 19;17(4):957. doi: 10.3390/ma17040957. Materials (Basel). 2024. PMID: 38399207 Free PMC article.
Cited by
-
Structure and photocatalytic performance of rice husk-like Ba-doped GaOOH under light irradiation.RSC Adv. 2019 Jun 26;9(35):19930-19939. doi: 10.1039/c9ra03670a. eCollection 2019 Jun 25. RSC Adv. 2019. PMID: 35514685 Free PMC article.
-
Preparation and Property Characterization of In2YSbO7/BiSnSbO6 Heterojunction Photocatalyst toward Photocatalytic Degradation of Indigo Carmine within Dye Wastewater under Visible-Light Irradiation.Materials (Basel). 2022 Sep 25;15(19):6648. doi: 10.3390/ma15196648. Materials (Basel). 2022. PMID: 36233988 Free PMC article.
References
-
- Zhe L., Shirley A.S., Thomas E.P., Amila O.D.S. Occurrence and fate of substituted diphenylamine antioxidants and benzotriazole UV stabilizers in various Canadian wastewater treatment processes. Water Res. 2017;124:158–166. - PubMed
-
- Liu R., Ruan T., Wang T., Song S., Guo F., Jiang G. Determination of nine benzotriazole UV stabilizers in environmental water samples by automated on-line solid phase extraction coupled with high-performance liquid chromatography−tandem mass spectrometry. Talanta. 2014;120:158–166. doi: 10.1016/j.talanta.2013.10.041. - DOI - PubMed
-
- Jia Y., Bakken L.R., Breedveld G.D., Aagaard P., Frostegård Å. Organic compounds that reach subsoil may threaten groundwater quality: Effect of benzotriazole on degradation kinetics and microbial community composition. Soil Biol. Biochem. 2006;38:2543–2556. doi: 10.1016/j.soilbio.2006.03.010. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

