NF-κB Activation in Lymphoid Malignancies: Genetics, Signaling, and Targeted Therapy
- PMID: 29587428
- PMCID: PMC6027339
- DOI: 10.3390/biomedicines6020038
NF-κB Activation in Lymphoid Malignancies: Genetics, Signaling, and Targeted Therapy
Abstract
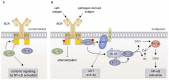
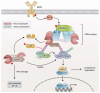
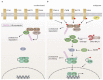
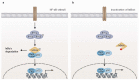
The NF-κB transcription factor family plays a crucial role in lymphocyte proliferation and survival. Consequently, aberrant NF-κB activation has been described in a variety of lymphoid malignancies, including diffuse large B-cell lymphoma, Hodgkin lymphoma, and adult T-cell leukemia. Several factors, such as persistent infections (e.g., with Helicobacter pylori), the pro-inflammatory microenvironment of the cancer, self-reactive immune receptors as well as genetic lesions altering the function of key signaling effectors, contribute to constitutive NF-κB activity in these malignancies. In this review, we will discuss the molecular consequences of recurrent genetic lesions affecting key regulators of NF-κB signaling. We will particularly focus on the oncogenic mechanisms by which these alterations drive deregulated NF-κB activity and thus promote the growth and survival of the malignant cells. As the concept of a targeted therapy based on the mutational status of the malignancy has been supported by several recent preclinical and clinical studies, further insight in the function of NF-κB modulators and in the molecular mechanisms governing aberrant NF-κB activation observed in lymphoid malignancies might lead to the development of additional treatment strategies and thus improve lymphoma therapy.
Keywords: CARD11; CARMA1; CD79; MyD88; NF-κB; leukemia; lymphoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

