Harnessing the Power of T Cells: The Promising Hope for a Universal Influenza Vaccine
- PMID: 29587436
- PMCID: PMC6027237
- DOI: 10.3390/vaccines6020018
Harnessing the Power of T Cells: The Promising Hope for a Universal Influenza Vaccine
Abstract
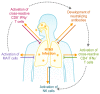
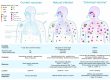
Next-generation vaccines that utilize T cells could potentially overcome the limitations of current influenza vaccines that rely on antibodies to provide narrow subtype-specific protection and are prone to antigenic mismatch with circulating strains. Evidence from animal models shows that T cells can provide heterosubtypic protection and are crucial for immune control of influenza virus infections. This has provided hope for the design of a universal vaccine able to prime against diverse influenza virus strains and subtypes. However, multiple hurdles exist for the realisation of a universal T cell vaccine. Overall primary concerns are: extrapolating human clinical studies, seeding durable effective T cell resident memory (Trm), population human leucocyte antigen (HLA) coverage, and the potential for T cell-mediated immune escape. Further comprehensive human clinical data is needed during natural infection to validate the protective role T cells play during infection in the absence of antibodies. Furthermore, fundamental questions still exist regarding the site, longevity and duration, quantity, and phenotype of T cells needed for optimal protection. Standardised experimental methods, and eventually simplified commercial assays, to assess peripheral influenza-specific T cell responses are needed for larger-scale clinical studies of T cells as a correlate of protection against influenza infection. The design and implementation of a T cell-inducing vaccine will require a consensus on the level of protection acceptable in the community, which may not provide sterilizing immunity but could protect the individual from severe disease, reduce the length of infection, and potentially reduce transmission in the community. Therefore, increasing the standard of care potentially offered by T cell vaccines should be considered in the context of pandemic preparedness and zoonotic infections, and in combination with improved antibody vaccine targeting methods. Current pandemic vaccine preparedness measures and ongoing clinical trials under-utilise T cell-inducing vaccines, reflecting the myriad questions that remain about how, when, where, and which T cells are needed to fight influenza virus infection. This review aims to bring together basic fundamentals of T cell biology with human clinical data, which need to be considered for the implementation of a universal vaccine against influenza that harnesses the power of T cells.
Keywords: T cell; influenza virus; universal vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
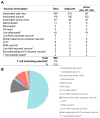
- Palache A., Abelin A., Hollingsworth R., Cracknell W., Jacobs C., Tsai T., Barbosa P., IFPMA Influenza Vaccine Supply (IFPMA IVS) task force Survey of distribution of seasonal influenza vaccine doses in 201 countries (2004–2015): The 2003 World Health Assembly resolution on seasonal influenza vaccination coverage and the 2009 influenza pandemic have had very little impact on improving influenza control and pandemic preparedness. Vaccine. 2017;35:4681–4686. - PubMed
-
- Xie H., Wan X.F., Ye Z., Plant E.P., Zhao Y., Xu Y., Li X., Finch C., Zhao N., Kawano T., et al. H3N2 Mismatch of 2014–15 Northern Hemisphere Influenza Vaccines and Head-to-head Comparison between Human and Ferret Antisera derived Antigenic Maps. Sci. Rep. 2015;5:15279. doi: 10.1038/srep15279. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

