Investigation of Linum flavum (L.) Hairy Root Cultures for the Production of Anticancer Aryltetralin Lignans
- PMID: 29587452
- PMCID: PMC5979590
- DOI: 10.3390/ijms19040990
Investigation of Linum flavum (L.) Hairy Root Cultures for the Production of Anticancer Aryltetralin Lignans
Abstract
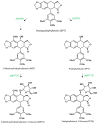
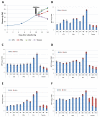
Linum flavum hairy root lines were established from hypocotyl pieces using Agrobacterium rhizogenes strains LBA 9402 and ATCC 15834. Both strains were effective for transformation but induction of hairy root phenotype was more stable with strain ATCC 15834. Whereas similar accumulation patterns were observed in podophyllotoxin-related compounds (6-methoxy-podophyllotoxin, podophyllotoxin and deoxypodophyllotoxin), significant quantitative variations were noted between root lines. The influence of culture medium and various treatments (hormone, elicitation and precursor feeding) were evaluated. The highest accumulation was obtained in Gamborg B5 medium. Treatment with methyl jasmonate, and feeding using ferulic acid increased the accumulation of aryltetralin lignans. These results point to the use of hairy root culture lines of Linum flavum as potential sources for these valuable metabolites as an alternative, or as a complement to Podophyllum collected from wild stands.
Keywords: 6-Methoxy-podophyllotoxin; Linum flavum; aryltetralin lignans; deoxypodophyllotoxin; hairy root; podophyllotoxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Koulman A., Quax W.J., Pras N. Podophyllotoxin and related lignans produced by plants. In: Ramawat K.G., editor. Biotechnology of Medicinal Plants: Vitalizer and Therapeutic. Science Publishers; New York, NY, USA: 2004. pp. 225–266.
-
- Holm B., Sehested M., Jensen P.B. Improved targeting of brain tumors using dexrazoxane rescue of topoisomerase II combined with supralethal doses of etoposide and teniposide. Clin. Cancer Res. 1998;4:1367–1373. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

