Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models
- PMID: 29587663
- PMCID: PMC5870823
- DOI: 10.1186/s12885-018-4238-4
Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models
Abstract
Background: Patient-derived tumor models are the new standard for pre-clinical drug testing and biomarker discovery. However, the emerging technology of primary pancreatic cancer organoids has not yet been broadly implemented in research, and complex organotypic models using organoids in co-culture with stromal and immune cellular components of the tumor have yet to be established. In this study, our objective was to develop and characterize pancreatic cancer organoids and multi-cell type organotypic co-culture models to demonstrate their applicability to the study of pancreatic cancer.
Methods: We employed organoid culture methods and flow cytometric, cytologic, immunofluorescent and immunohistochemical methods to develop and characterize patient-derived pancreatic cancer organoids and multi-cell type organotypic co-culture models of the tumor microenvironment.
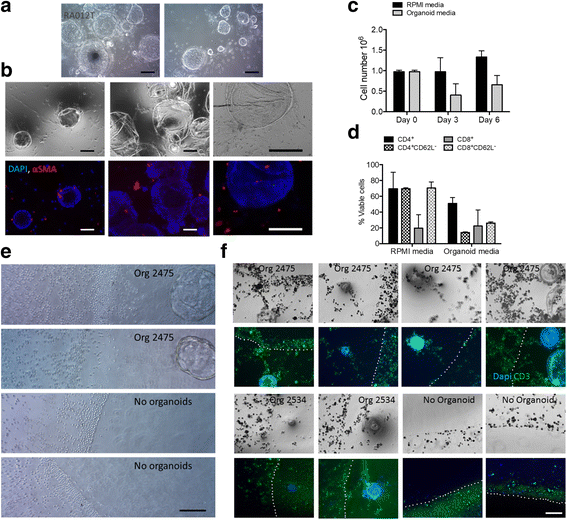
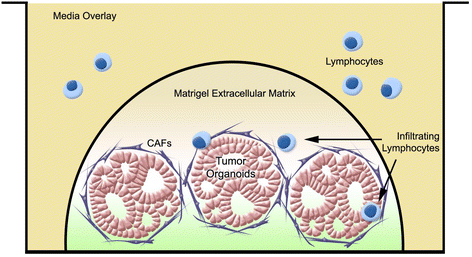
Results: We describe the culture and characterization of human pancreatic cancer organoids from resection, ascites and rapid autopsy sources and the derivation of adherent tumor cell monocultures and tumor-associated fibroblasts from these sources. Primary human organoids displayed tumor-like cellular morphology, tissue architecture and polarity in contrast to cell line spheroids, which formed homogenous, non-lumen forming spheres. Importantly, we demonstrate the construction of complex organotypic models of tumor, stromal and immune components of the tumor microenvironment. Activation of myofibroblast-like cancer associated fibroblasts and tumor-dependent lymphocyte infiltration were observed in these models.
Conclusions: These studies provide the first report of novel and disease-relevant 3D in-vitro models representing pancreatic tumor, stromal and immune components using primary organoid co-cultures representative of the tumor-microenvironment. These models promise to facilitate the study of tumor-stroma and tumor-immune interaction and may be valuable for the assessment of immunotherapeutics such as checkpoint inhibitors in the context of T-cell infiltration.
Keywords: CAFs; Microenvironment; Organoid; Organotypic culture; PDAC; Pancreatic Cancer; TILs; Tumor immunology; Tumor stroma.
Conflict of interest statement
Ethics approval and consent to participate
All procedures involving clinical samples are covered under IRB#PRO00012151 (MCW Surgical Biorepository) and PRO00028870 (preclinical chemosensitivity testing), reviewed by Medical College of Wisconsin/Froedtert Hospital Institutional Review Board #2. All patients provided written informed consent at the time of enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, Shi C, Danenberg K, Danenberg PV, Kuramochi H, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12(15):4652–4661. doi: 10.1158/1078-0432.CCR-06-0113. - DOI - PubMed
-
- Thomas RM, Truty MJ, Kim M, Kang Y, Zhang R, Chatterjee D, Katz MH, Fleming JB. The canary in the coal mine: the growth of patient-derived tumorgrafts in mice predicts clinical recurrence after surgical resection of pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2015;22(6):1884–1892. doi: 10.1245/s10434-014-4241-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

