Cost effectiveness analysis of afatinib versus pemetrexed-cisplatin for first-line treatment of locally advanced or metastatic EGFR mutation positive non-small-cell lung cancer from the Singapore healthcare payer's perspective
- PMID: 29587666
- PMCID: PMC5872570
- DOI: 10.1186/s12885-018-4223-y
Cost effectiveness analysis of afatinib versus pemetrexed-cisplatin for first-line treatment of locally advanced or metastatic EGFR mutation positive non-small-cell lung cancer from the Singapore healthcare payer's perspective
Abstract
Background: Non-small-cell lung cancer (NSCLC) accounts for 85% of all lung cancers and is associated with a poor prognosis. Afatinib is an irreversible ErbB family blocker recommended in clinical guidelines as a first-line treatment for NSCLC which harbours an epidermal growth factor receptor (EGFR) mutation. The objective of this study was to evaluate the cost-effectiveness of afatinib versus pemetrexed-cisplatin for first-line treatment of locally advanced or metastatic EGFR mutation positive NSCLC in Singapore.
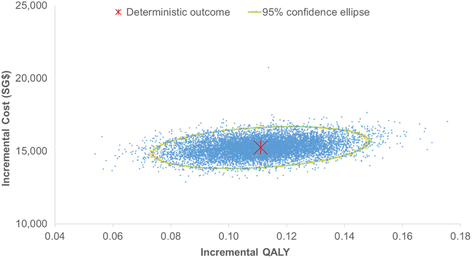
Methods: A partitioned survival model with three health states (progression-free, progressive disease and death) was developed from a healthcare payer perspective. Survival curves from the LUX-Lung 3 trial (afatinib versus pemetrexed-cisplatin chemotherapy) were extrapolated beyond the trial period to estimate the underlying progression-free survival and overall survival parametric distributions. Rates of adverse reactions were also estimated from LUX-Lung 3 while health utilities from overseas were derived from the literature in the absence of local estimates. Direct costs were sourced from public healthcare institutions in Singapore. Incremental cost-effectiveness ratios (ICERs) were calculated over a 5 year time horizon. Deterministic and probabilistic sensitivity analyses and additional scenario analyses were conducted to explore the impact of uncertainties and assumptions on the cost-effectiveness results.
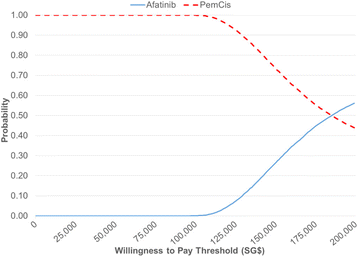
Results: In the base-case analysis, the ICER for afatinib versus pemetrexed-cisplatin was SG$137,648 per quality-adjusted life year (QALY) gained and SG$109,172 per life-year gained. One-way sensitivity analysis showed the ICER was most sensitive to variations in the utility values, the cost of afatinib and time horizon. Scenario analyses showed that even reducing the cost of afatinib by 50% led to a high ICER which was unlikely to represent a cost-effective use of healthcare resources.
Conclusions: Compared with pemetrexed-cisplatin, afatinib is not cost-effective as a first-line treatment for advanced EGFR mutation-positive NSCLC in Singapore. The findings from our study will be useful to inform local healthcare decision-making and resource allocations for NSCLC treatments, together with other considerations such as clinical effectiveness, safety and affordability of TKIs.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Cost-Effectiveness and Value of Information of Erlotinib, Afatinib, and Cisplatin-Pemetrexed for First-Line Treatment of Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer in the United States.Value Health. 2015 Sep;18(6):774-82. doi: 10.1016/j.jval.2015.04.008. Epub 2015 Jun 22. Value Health. 2015. PMID: 26409604
-
Cost-effectiveness analysis of osimertinib for first-line treatment of locally advanced or metastatic EGFR mutation positive non-small cell lung cancer in Singapore.J Med Econ. 2020 Nov;23(11):1330-1339. doi: 10.1080/13696998.2020.1819822. Epub 2020 Sep 21. J Med Econ. 2020. PMID: 32886557
-
EGFR mutation-guided use of afatinib, erlotinib and gefitinib for advanced non-small-cell lung cancer in Hong Kong - A cost-effectiveness analysis.PLoS One. 2021 Mar 1;16(3):e0247860. doi: 10.1371/journal.pone.0247860. eCollection 2021. PLoS One. 2021. PMID: 33647045 Free PMC article.
-
Pemetrexed for the maintenance treatment of locally advanced or metastatic non-small cell lung cancer.Health Technol Assess. 2010 Oct;14(Suppl. 2):33-9. doi: 10.3310/hta14suppl2/05. Health Technol Assess. 2010. PMID: 21047489 Review.
-
Gefitinib for the first-line treatment of locally advanced or metastatic non-small cell lung cancer.Health Technol Assess. 2010 Oct;14(Suppl. 2):71-9. doi: 10.3310/hta14suppl2/10. Health Technol Assess. 2010. PMID: 21047494 Review.
Cited by
-
Cost-Effectiveness of 12 First-Line Treatments for Patients With Advanced EGFR Mutated NSCLC in the United Kingdom and China.Front Oncol. 2022 Jun 6;12:819674. doi: 10.3389/fonc.2022.819674. eCollection 2022. Front Oncol. 2022. PMID: 35785198 Free PMC article.
-
Modelled Economic Analysis for Dacomitinib-A Cost Effectiveness Analysis in Treating Patients With EGFR-Mutation-Positive Non-Small Cell Lung Cancer in China.Front Oncol. 2021 Dec 14;11:564234. doi: 10.3389/fonc.2021.564234. eCollection 2021. Front Oncol. 2021. PMID: 34970476 Free PMC article.
-
Comparative effectiveness and cost-effectiveness of three first-line EGFR-tyrosine kinase inhibitors: Analysis of real-world data in a tertiary hospital in Taiwan.PLoS One. 2020 Apr 8;15(4):e0231413. doi: 10.1371/journal.pone.0231413. eCollection 2020. PLoS One. 2020. PMID: 32267879 Free PMC article.
-
Cost-effectiveness of ribociclib as initial treatment for premenopausal women with advanced breast cancer in Singapore.Cancer Rep (Hoboken). 2021 Feb;4(1):e1308. doi: 10.1002/cnr2.1308. Epub 2020 Oct 21. Cancer Rep (Hoboken). 2021. PMID: 33085843 Free PMC article.
-
Critical Examination of Modeling Approaches Used in Economic Evaluations of First-Line Treatments for Locally Advanced or Metastatic Non-Small Cell Lung Cancer Harboring Epidermal Growth Factor Receptor Mutations: A Systematic Literature Review.Pharmacoeconomics. 2024 May;42(5):527-568. doi: 10.1007/s40273-024-01362-2. Epub 2024 Mar 15. Pharmacoeconomics. 2024. PMID: 38489077 Free PMC article.
References
-
- Singapore Cancer Registry Annual Registry Report 2015. Released 19 June 2017. https://www.nrdo.gov.sg/docs/librariesprovider3/Publications-Cancer/canc.... Accessed 20 July 2017.
-
- Besse B, Adjei A, Baas P, Meldgaard P, Nicolson M, Paz-Ares L, Reck M, Smit EF, Syrigos K, Stahel R, et al. 2nd ESMO consensus conference on lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol. 2014;25:1475–1484. doi: 10.1093/annonc/mdu123. - DOI - PubMed
-
- Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G, Yang PC. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154–162. doi: 10.1097/JTO.0000000000000033. - DOI - PMC - PubMed
-
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

