A hybrid type I randomized effectiveness-implementation trial of patient navigation to improve access to services for children with autism spectrum disorder
- PMID: 29587698
- PMCID: PMC5870193
- DOI: 10.1186/s12888-018-1661-7
A hybrid type I randomized effectiveness-implementation trial of patient navigation to improve access to services for children with autism spectrum disorder
Abstract
Background: Significant racial, ethnic, and socioeconomic disparities exist in access to evidence-based treatment services for children with autism spectrum disorder (ASD). Patient Navigation (PN) is a theory-based care management strategy designed to reduce disparities in access to care. The purpose of this study is to test the effectiveness of PN a strategy to reduce disparities in access to evidence-based services for vulnerable children with ASD, as well as to explore factors that impact implementation.
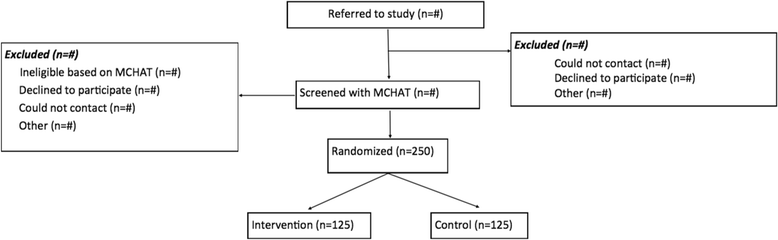
Methods: This study uses a hybrid type I randomized effectiveness/implementation design to test effectiveness and collect data on implementation concurrently. It is a two-arm comparative effectiveness trial with a target of 125 participants per arm. Participants are families of children age 15-27 months who receive a positive screen for ASD at a primary care visit at urban clinics in Massachusetts (n = 6 clinics), Connecticut (n = 1), and Pennsylvania (n = 2). The trial measures diagnostic interval (number of days from positive screen to diagnostic determination) and time to receipt of evidence-based ASD services/recommended services (number of days from date of diagnosis to receipt of services) in those with PN compared to and activated control -Conventional Care Management - which is similar to care management received in a high quality medical home. At the same time, a mixed-method implementation evaluation is being carried out.
Discussion: This study will examine the effectiveness of PN to reduce the time to and receipt of evidence-based services for vulnerable children with ASD, as well as factors that influence implementation. Findings will tell us both if PN is an effective approach for improving access to evidence-based care for children with ASD, and inform future strategies for dissemination.
Trial registration: NCT02359084 Registered February 1, 2015.
Keywords: Autism spectrum disorder; Disparities; Navigation.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the institutional review board at Boston University (protocol number H-33008
Consent for publication
All participants were clearly informed about the purpose of the project and the planned use of the resulting data, including publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Tonge BJ, Bull K, Brereton A, Wilson R. A review of evidence-based early intervention for behavioural problems in children with autism spectrum disorder: the core components of effective programs, child-focused interventions and comprehensive treatment models. Curr Opin Psychiatry. 2014;27(2):158–165. - PubMed
-
- Weitlauf AS, McPheeters ML, Peters B, et al. Therapies for Children With Autism Spectrum Disorder: Behavioral Interventions Update. Comparative Effectiveness Review No. 137. (Prepared by the Vanderbilt Evidence-based Practice Center under Contract No. 290-2012-00009-I.) AHRQ Publication No. 14-EHC036-EF. Rockville: Agency for Healthcare Research and Quality; 2014. www.effectivehealthcare.ahrq.gov/reports/final.cfm. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical