Clinical outcome after particle therapy for meningiomas of the skull base: toxicity and local control in patients treated with active rasterscanning
- PMID: 29587795
- PMCID: PMC5870393
- DOI: 10.1186/s13014-018-1002-5
Clinical outcome after particle therapy for meningiomas of the skull base: toxicity and local control in patients treated with active rasterscanning
Abstract
Background: Meningiomas of the skull base account for 25-30% of all meningiomas. Due to the complex structure of the cranial base and its close proximity to critical structures, surgery is often associated with substantial morbidity. Treatment options include observation, aggressive surgical intervention, stereotactic or conventional radiotherapy. In this analysis we evaluate the outcome of 110 patients with meningiomas of the skull base treated with particle therapy. It was performed within the framework of the "clinical research group heavy ion therapy" and supported by the German Research Council (DFG, KFO 214).
Methods: Between May 2010 and November 2014, 110 Patients with skull base meningioma were treated with particle radiotherapy at the Heidelberg Ion Therapy Center (HIT). Primary localizations included the sphenoid wing (n = 42), petroclival region (n = 23), cavernous sinus (n = 4), sella (n = 10) and olfactory nerve (n = 4). Sixty meningiomas were benign (WHO °I); whereas 8 were high-risk (WHO °II (n = 7) and °III (n = 1)). In 42 cases histology was not examined, since no surgery was performed. Proton (n = 104) or carbon ion (n = 6) radiotherapy was applied at Heidelberg Ion Therapy Center (HIT) using raster-scanning technique for active beam delivery. Fifty one patients (46.4%) received radiotherapy due to tumor progression, 17 (15.5%) after surgical resection and 42 (38.2%) as primary treatment.
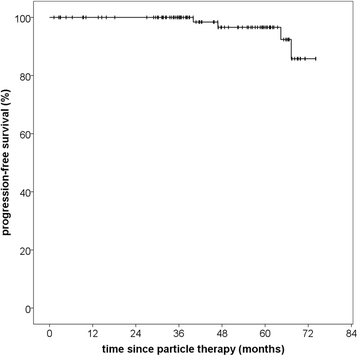
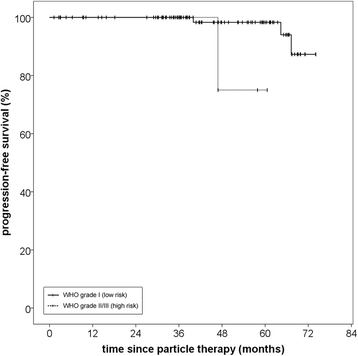
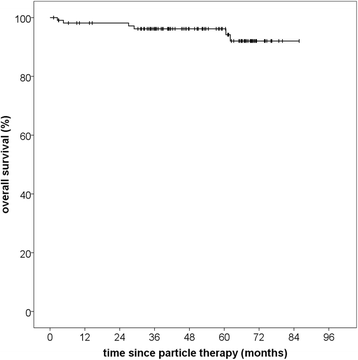
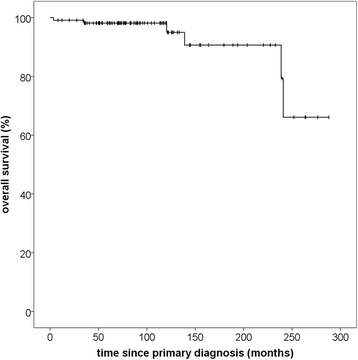
Results: Median follow-up in this analysis was 46,8 months (95% CI 39,9-53,7; Q1-Q3 34,3-61,7). Particle radiotherapy could be performed safely without toxicity-related interruptions. No grade IV or V toxicities according to CTCAE v4.0 were observed. Particle RT offered excellent overall local control rates with 100% progression-free survival (PFS) after 36 months and 96.6% after 60 months. Median PFS was not reached due to the small number of events. Histology significantly impacted PFS with superior PFS after 5 years for low-risk tumors (96.6% vs. 75.0%, p = 0,02). Overall survival was 96.2% after 60 months and 92.0% after 72 months from therapy. Of six documented deaths, five were definitely not and the sixth probably not meningioma-related.
Conclusion: Particle radiotherapy is an excellent treatment option for patients with meningiomas of the skull base and can lead to long-term tumor control with minimal side effects. Other prospective studies with longer follow-up will be necessary to further confirm the role of particle radiotherapy in skull base meningioma.
Keywords: Active raster-scanning; Benign; Carbon ion therapy; Malignant; Proton therapy; Quality of life; Radiotherapy; Radiotolerance; Skull base; Toxicity.
Conflict of interest statement
Ethics approval and consent to participate
The Heidelberg Ethics Committee approved this study on the 9th of July 2013 (S-207/2013).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Combs SE, Edler L, Burkholder I, Rieken S, Habermehl D, Jäkel O, et al. Treatment of patients with atypical meningiomas Simpson grade 4 and 5 with a carbon ion boost in combination with postoperative photon radiotherapy: the MARCIE trial. BMC Cancer. 2010;10:615. doi: 10.1186/1471-2407-10-615. - DOI - PMC - PubMed
-
- Combs SE, Adeberg S, Dittmar J-O, Welzel T, Rieken S, Habermehl D, et al. Skull base meningiomas: long-term results and patient self-reported outcome in 507 patients treated with fractionated stereotactic radiotherapy (FSRT) or intensity modulated radiotherapy (IMRT) Radiother Oncol. 2013;106:186–191. doi: 10.1016/j.radonc.2012.07.008. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

