Clinical and MRI remission in patients with nonradiographic axial spondyloarthritis who received long-term open-label adalimumab treatment: 3-year results of the ABILITY-1 trial
- PMID: 29587851
- PMCID: PMC5870399
- DOI: 10.1186/s13075-018-1556-5
Clinical and MRI remission in patients with nonradiographic axial spondyloarthritis who received long-term open-label adalimumab treatment: 3-year results of the ABILITY-1 trial
Abstract
Background: Adalimumab was effective in treating patients with nonradiographic axial spondyloarthritis (nr-axSpA) in the 12-week ABILITY-1 trial. We present long-term efficacy and safety results of adalimumab from the open-label ABILITY-1 extension, including the relationship between clinical and magnetic resonance imaging (MRI) remission and impact of sustained clinical remission on physical function.
Methods: Patients received adalimumab 40 mg every other week or placebo for 12 weeks, then open-label adalimumab for up to 144 weeks. Clinical and safety data were collected through 3 years, and MRI data were collected until 2 years. Analyses were performed in the total population and subpopulation with positive MRI and/or elevated C-reactive protein (MRI/CRP-positive) at baseline. Clinical and MRI remission definitions included Ankylosing Spondylitis Disease Activity Score inactive disease (ASDAS ID; score < 1.3) and Spondyloarthritis Research Consortium of Canada (SPARCC) MRI score < 2 for sacroiliac joints (SIJs), spine, or both. Physical function was assessed using the Bath Ankylosing Spondylitis Functional Index.
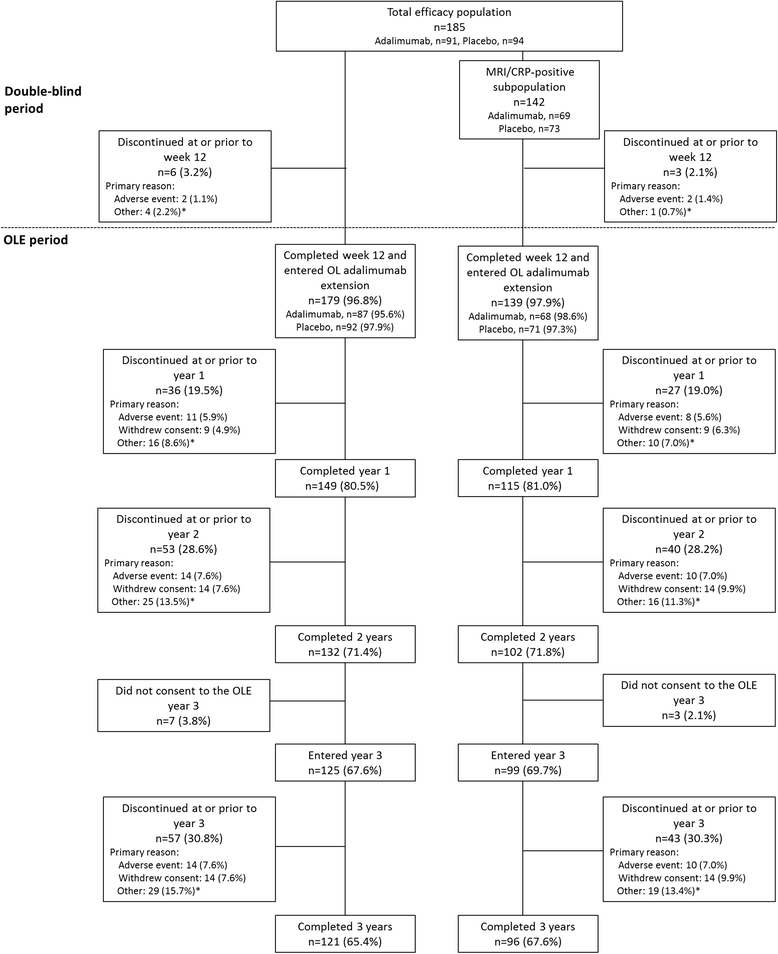
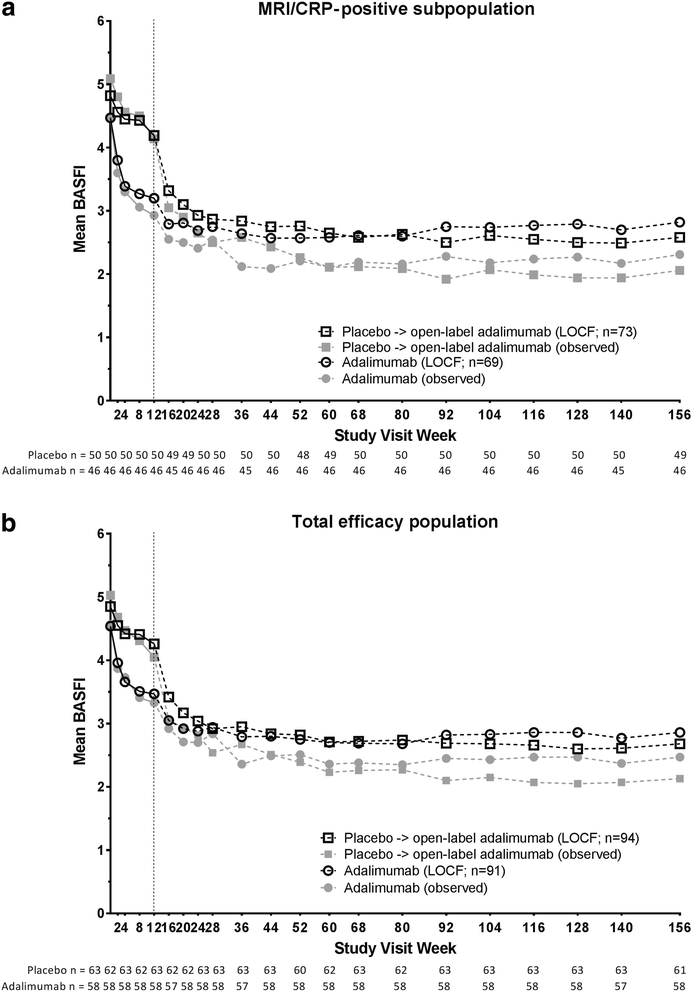
Results: Overall, 185 patients were included in the total population and 142 in the MRI/CRP-positive subpopulation; 65% and 68%, respectively, completed 3 years. Clinical, functional, and MRI improvements were similar and equally sustainable in both populations. At year 3, the percentages of patients in ASDAS ID in the MRI/CRP-positive subpopulation were 30%/33% (nonresponder imputation) and 46%/49% (observed) for those initially receiving adalimumab/placebo. At years 1 and 2, patients in ASDAS ID vs not had significantly greater improvements in SPARCC SIJ scores from baseline (P < 0.001). Among patients with baseline MRI scores ≥ 2 who achieved ASDAS ID at year 2, 44-68% also had MRI remission. Significantly more patients with sustained ASDAS ID through year 2 or 3 vs without achieved normal physical function (100% vs 48%; 100% vs 44%; both P < 0.001). No new safety concerns were observed.
Conclusions: In the ABILITY-1 study of nr-axSpA, adalimumab therapy provided sustained clinical and functional improvements through 3 years, as well as suppression of MRI axial inflammation, which was greater in patients who achieved clinical remission. Sustained clinical remission was associated with increased attainment of normal physical function. The safety profile of adalimumab was consistent with prior studies.
Trial registration: ClinicalTrials.gov , NCT00939003 ; registered on July 10, 2009.
Keywords: Adalimumab; Anti-TNF; Axial spondyloarthritis.
Conflict of interest statement
Ethics approval and consent to participate
This study was conducted in accordance with the International Conference on Harmonization good clinical practice guideline and the Declaration of Helsinki. Approval of an institutional ethics review board and voluntary written informed patient consent were obtained prior to initiation of study procedures.
Consent for publication
Not applicable.
Competing interests
DvdH has received consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, and UCB and is director of Imaging Rheumatology B.V. JS has received research grants, consulting fees, and speaker’s fees from AbbVie, Merck, Pfizer, and UCB. WPM has received research grants and consulting fees from AbbVie, Amgen, Eli Lilly, Janssen, Merck, Novartis, Pfizer, SYNARC, and UCB and is chief medical officer of CaRE Arthritis. RGL has received consulting or advisory board fees from AbbVie, BioClinica, Janssen, Parexel, and UCB. SC, MH, JKA, and ALP are full-time employees of AbbVie and may hold stock and/or options.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, Braun J, Chou CT, Collantes-Estevez E, Dougados M, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–783. doi: 10.1136/ard.2009.108233. - DOI - PubMed
-
- van der Heijde D, Machado P, Braun J, Hermann KG, Baraliakos X, Hsu B, Baker D, Landewé R. MRI inflammation at the vertebral unit only marginally predicts new syndesmophyte formation: a multilevel analysis in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71(3):369–373. doi: 10.1136/annrheumdis-2011-200208. - DOI - PubMed
-
- Poddubnyy D, Gaydukova I, Hermann KG, Song IH, Haibel H, Braun J, Sieper J. Magnetic resonance imaging compared to conventional radiographs for detection of chronic structural changes in sacroiliac joints in axial spondyloarthritis. J Rheumatol. 2013;40(9):1557–1565. doi: 10.3899/jrheum.130141. - DOI - PubMed
-
- Hermann KG, Baraliakos X, van der Heijde DM, Jurik AG, Landewé R, Marzo-Ortega H, Ostergaard M, Rudwaleit M, Sieper J, Braun J, et al. Descriptions of spinal MRI lesions and definition of a positive MRI of the spine in axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI study group. Ann Rheum Dis. 2012;71(8):1278–1288. doi: 10.1136/ard.2011.150680. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

