[Observation - An Favorable Option Forthoracic Dissemination Patients with Lung Adenocarcinoma or Squamous Carcinoma]
- PMID: 29587911
- PMCID: PMC5973350
- DOI: 10.3779/j.issn.1009-3419.2018.04.14
[Observation - An Favorable Option Forthoracic Dissemination Patients with Lung Adenocarcinoma or Squamous Carcinoma]
Abstract
Background: Surgery was not standard-of-care of patients with advanced lung cancer. However, a serial of retrospective studies demonstrated that thoracic dissemination (M1a) patients could benefit from contraindicated surgery. After non-standard treatment, how should these patients choose following treatment approaches? Herein, we conducted this retrospective study to explore subsequent optimal treatment approaches.
Methods: Different therapeutic approaches were evaluated by comparing progression-free survival (PFS), overall survival (OS), time to treatment interval (TTI) using the Kaplan-Meier method and Log-rank test. A Cox proportional hazards regression model was used for multivariate analysis.
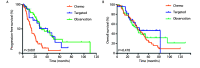
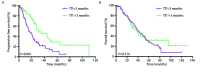
Results: 141 eligible were enrolled. The median PFS of chemotherapy group, targeted therapy group and observation group were 14.7, 41.0 and 31.0 months, respectively (95%CI: 19.01-26.01; P<0.001). There was no significantly statistically difference between median PFS of targeted group and observation group (P=0.006). The median OS were 39.0, 42.6 and 38.1 months (95%CI: 32.47-45.33; P=0.478). The median PFS and OS of TTI<3 months and TTI ≥3 months were 15.2 months versus 31.0 months (95%CI: 19.01-26.06; P<0.001) and 41.7 months versus 38.7 months (95%CI: 32.47-45.33; P=0.714). Multivariate analyses revealed gender (P=0.027), lymph node status (P=0.036) and initial therapy (P<0.001) were independent prognostic factors for PFS.
Conclusions: Observation did not shorten survival of thoracic dissemination patients with lung adenocarcinoma or squamous carcinoma, therefore, it could be an favorable option. But prospective randomized controlled study was needed to confirm its validity.
背景与目的 手术非晚期期患者治疗的标准治疗,但是大量的回顾性研究显示胸腔内播散型肺癌接受主病灶切除后获益明显。非标准治疗之后患者该选择何种治疗策略?本研究通过回顾性数据去探究接受了主病灶切除的胸腔内播散型肺癌患者接下来何总治疗方式更优。方法 回顾性收集早期肺腺癌或肺鳞癌且复发模式为胸腔内播散型患者;或拟行肺癌根治术,但术中胸腔探查发现胸腔内播散,接受主病灶切除的肺腺癌或肺鳞癌患者的一般资料、病理、淋巴结状态、基因突变状态、初始治疗方式等,随访至进展、死亡或失访,记录患者无进展生存时间、总生存时间、从确诊到开始治疗的时间。通过Kaplan-Meier绘制生存曲线,Log-rank检验比较组间生存差异,Cox比例回归风险模型分析无进展生存期(progression-free survival, PFS)和总生存期(overall survival, OS)相关预后因子。结果 研究共纳入141例患者,70例r-M1a和71例s-M1a1患者。化疗组、靶向组、随访观察组患者中位PFS分别是 14.7个月、41.0个月和31.0个月(95%CI: 19.01-26.01; P<0.001),靶向治疗组和随访观察组患者PFS差异无统计学意义(P=0.600)。中位OS分别为39.0个月、42.6个月和38.1个月(95%CI: 32.47-45.33; P=0.478)。TTI<3个月组和TTI≥3个月组患者的中位PFS分别是15.2个月和31.0个月(95%CI: 19.01-26.06; P<0.001),中位OS分别是41.7个月和38.7个月(95%CI: 32.47-45.33; P=0.714)。多因素分析显示性别(P=0.027)、淋巴结状态(P=0.036)、初始治疗方式(P<0.001)是PFS独立预后因子。结论 随访观察不会缩短胸腔内播散腺癌和鳞癌患者的生存时间,是一种可选的治疗策略。.
Keywords: Adenocarcinoma; Observation; Squamous carcinoma; Thoracic dissemination.
Figures

Similar articles
-
Extracapsular extension is a powerful prognostic factor in stage IIA-IIIA non-small cell lung cancer patients with completely resection.Int J Clin Exp Pathol. 2015 Sep 1;8(9):11268-77. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26617851 Free PMC article.
-
Optimizing Survival of Patients With Marginally Operable Stage IIIA Non-Small-Cell Lung Cancer Receiving Chemoradiotherapy With or Without Surgery.Clin Lung Cancer. 2016 Nov;17(6):550-557. doi: 10.1016/j.cllc.2016.05.013. Epub 2016 Jun 8. Clin Lung Cancer. 2016. PMID: 27378175 Clinical Trial.
-
Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial.Lancet Oncol. 2014 Feb;15(2):143-55. doi: 10.1016/S1470-2045(13)70586-2. Epub 2014 Jan 9. Lancet Oncol. 2014. PMID: 24411639 Clinical Trial.
-
Carcinoma of deep male urethra.Urology. 1984 Dec;24(6):527-31. doi: 10.1016/0090-4295(84)90095-5. Urology. 1984. PMID: 6390925 Review.
-
Comparative efficacy analysis identifies immune checkpoint blockade as a new survival benchmark in advanced cutaneous squamous cell carcinoma.Eur J Cancer. 2022 Jul;170:42-53. doi: 10.1016/j.ejca.2022.03.035. Epub 2022 May 17. Eur J Cancer. 2022. PMID: 35594611
Cited by
-
Development of a Prognostic Nomogram for Patients with Lung Adenocarcinoma in the Stages I, II, and III Based on Immune Scores.Int J Gen Med. 2021 Nov 23;14:8677-8688. doi: 10.2147/IJGM.S337934. eCollection 2021. Int J Gen Med. 2021. PMID: 34849011 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

